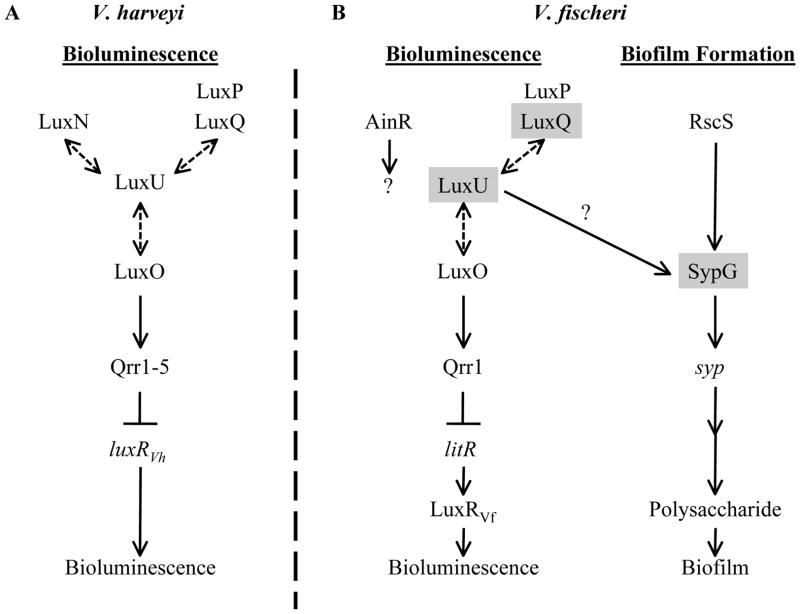
Fig. 1. Models for the regulation by the Lux and Syp pathways.
(A) In V. harveyi, bioluminescence is regulated by the Lux phosphorelay, composed of the sensor kinases (SKs) LuxQ (which interacts with the periplasmic protein LuxP), LuxN, and CqsS (not depicted), the phosphotransferase LuxU, and the response regulator (RR) LuxO. Phosphoryl-transfer (dashed, double-sided arrows) occurs between the SKs, LuxU, and LuxO. Under low cell density conditions, LuxO is phosphorylated by the kinase activity of the SKs and activates transcription of the qrr sRNAs, which bind to the transcript of luxRVH and prevent its translation. Without LuxRVH, the lux operon (not depicted) is not expressed and light (bioluminescence) is not produced. At high cell densities, LuxO is dephosphorylated by the phosphatase activity of the SKs, leading to subsequent production of LuxRVH and bioluminescence. Autoinducers (AIs) or the AI synthases (LuxM, LuxS, or CqsA) are not depicted for simplicity.
(B) In V. fischeri, the Lux phosphorelay functions in largely the same manner to regulate bioluminescence, with homologs of LuxP, LuxQ, LuxU, and LuxO, but not CqsS or LuxN; V. fischeri encodes another putative SK, AinR. Additionally, V. fischeri uses the LuxRVH homolog, LitR, to activate transcription of LuxRVF (not similar to LuxRVH), which promotes transcription of the lux operon (when bound to the AI produced by LuxI) (not depicted), leading to subsequent light production (bioluminescence). Regulators shaded in gray indicate those found in this study to be involved in biofilm formation. RscS is an SK known to control biofilm formation. Phosphorylation of the RR SypG is predicted to activate transcription of the syp locus, which encodes proteins thought to regulate, produce, and transport a polysaccharide necessary for biofilm formation. The specific activity of LuxU in activating biofilm formation is unknown, but it appears from the current study to work at or above the level of syp transcription, likely at the level of SypG activation (indicated by a question mark). This figure is adapted from (Visick, 2005).