Abstract
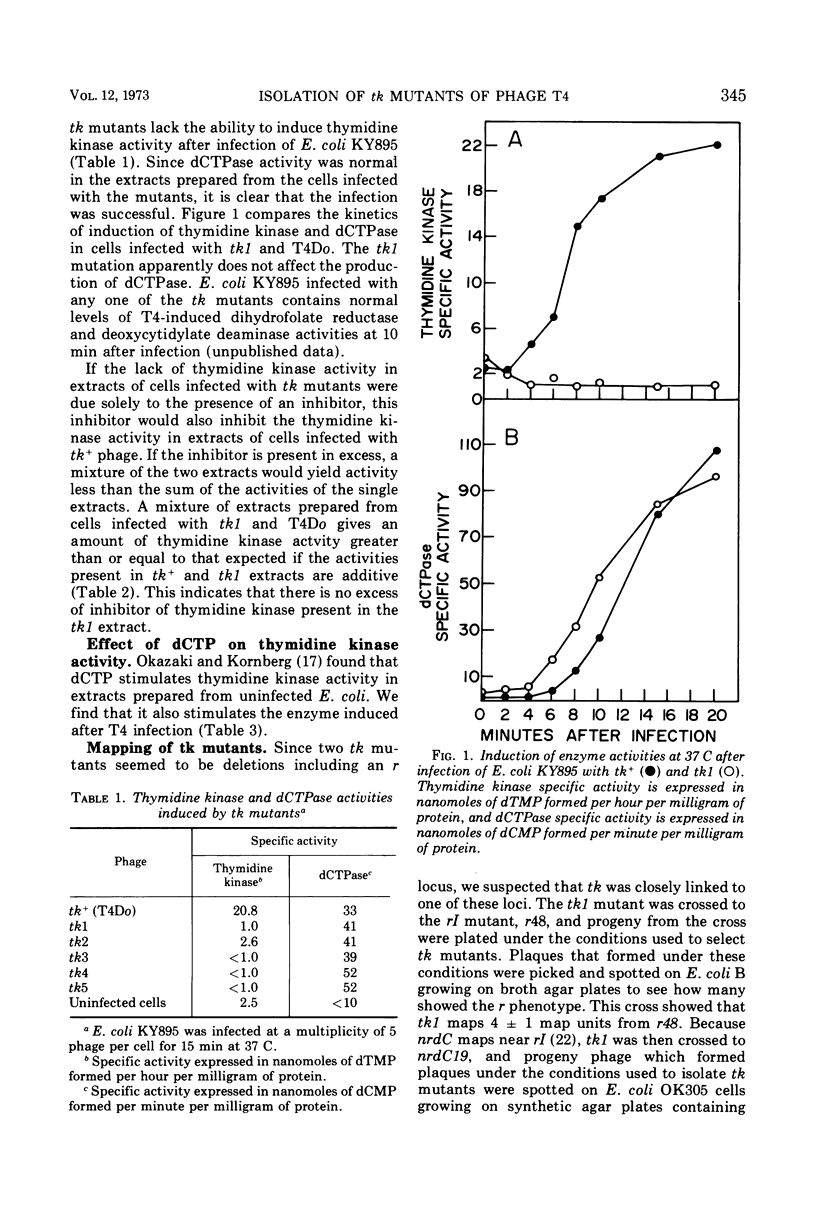
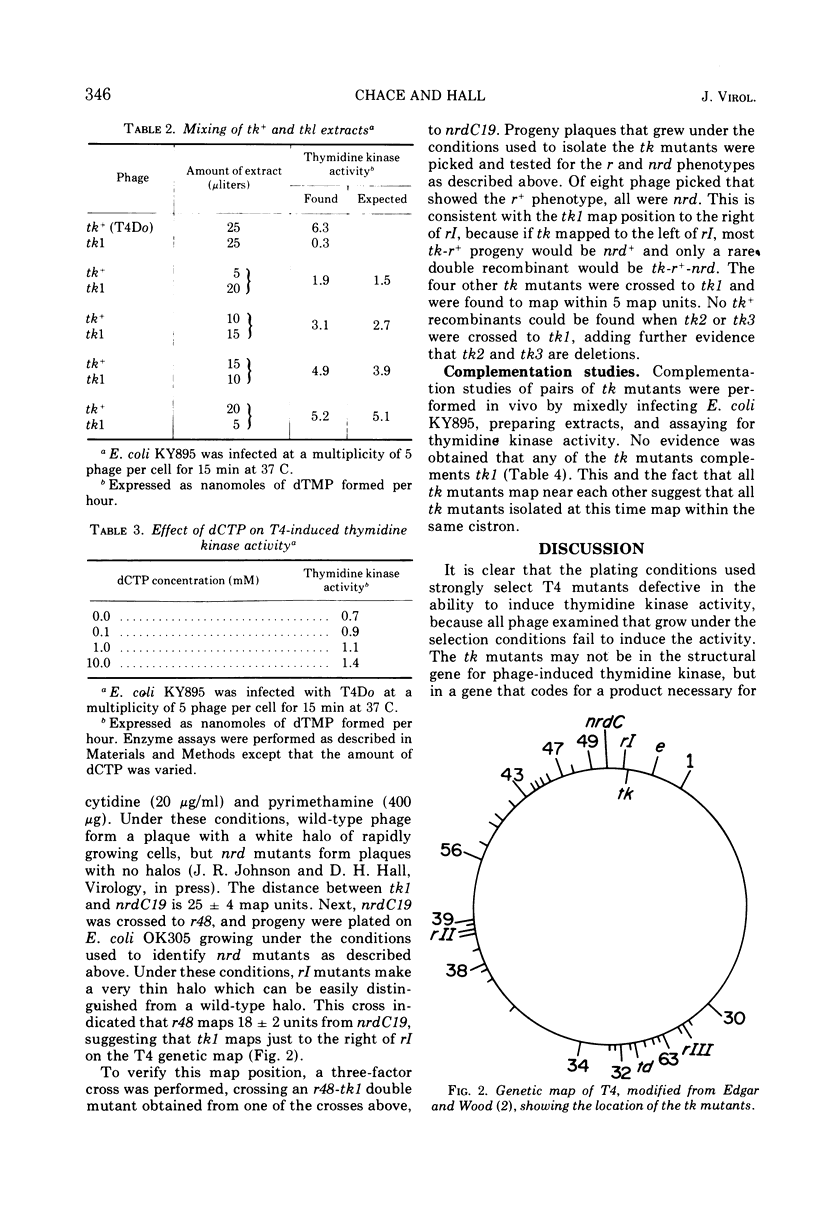
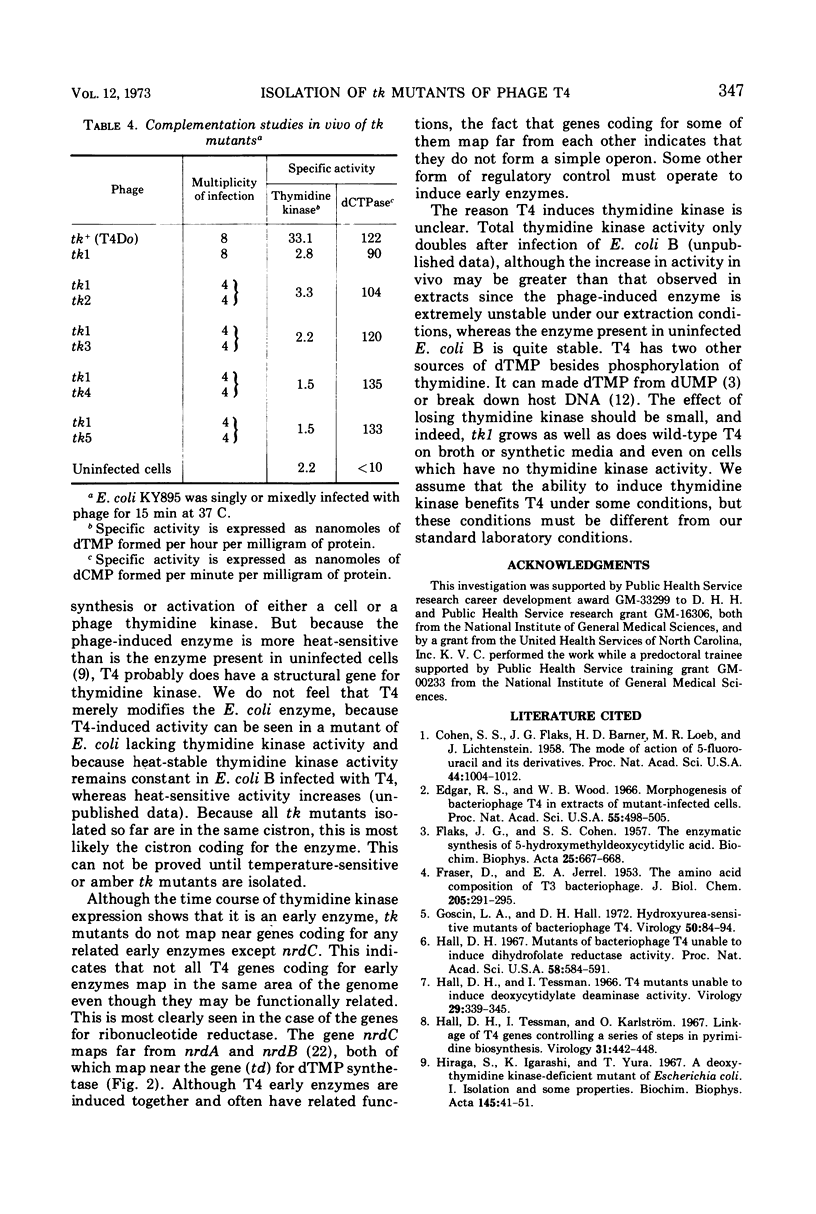
New mutants of T4 have been isolated by using a strain of Escherichia coli lacking thymidine kinase activity. These T4 mutants, designated tk, are able to grow on this E. coli strain under light on plates containing 5-bromodeoxyuridine and were all found to be unable to induce thymidine kinase (ATP: thymidine 5′-phosphotransferase, EC 2.7.1.21). All of these tk mutants fall into one complementation group which maps just to the right of rI on the standard T4 genetic map, far from most other genes coding for enzymes involved in pyrimidine metabolism. The tk mutants grow as well as wild-type T4, indicating that thymidine kinase is a non-essential enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAKS J. G., COHEN S. S. The enzymic synthesis of 5-hydroxymethyldeoxycytidylic acid. Biochim Biophys Acta. 1957 Sep;25(3):667–668. doi: 10.1016/0006-3002(57)90553-x. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Goscin L. A., Hall D. H. Hydroxyurea-sensitive mutants of bacteriophage T4. Virology. 1972 Oct;50(1):84–94. doi: 10.1016/0042-6822(72)90348-0. [DOI] [PubMed] [Google Scholar]
- Hall D. H. Mutants of bacteriophage T4 unable to induce dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1967 Aug;58(2):584–591. doi: 10.1073/pnas.58.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Tessman I., Karlström O. Linkage of T4 genes controlling a series of steps in pyrimidine biosynthesis. Virology. 1967 Mar;31(3):442–448. doi: 10.1016/0042-6822(67)90224-3. [DOI] [PubMed] [Google Scholar]
- Hall D. H., Tessman I. T4 mutants unable to induce deoxycytidylate deaminase activity. Virology. 1966 Jun;29(2):339–345. doi: 10.1016/0042-6822(66)90041-9. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Igarashi K., Yura T. A deoxythymidine kinase-deficient mutant of Escherichia coli. I. Isolation and some properties. Biochim Biophys Acta. 1967 Aug 22;145(1):41–51. doi: 10.1016/0005-2787(67)90652-1. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Hiraga S., Yura T. A deoxythymidine kinase deficient mutant of Escherichia coli. II. Mapping and transduction studies with phage phi 80. Genetics. 1967 Nov;57(3):643–654. doi: 10.1093/genetics/57.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- KOZLOFF L. M. Origin and fate of bacteriophage material. Cold Spring Harb Symp Quant Biol. 1953;18:209–220. doi: 10.1101/sqb.1953.018.01.032. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lion M. B. Search for a mechanism for the increased sensitivity of 5-bromouracil-substituted DNA to ultraviolet radiation. II. Single-strand breaks in the DNA of irradiated 5-bromouracil-substituted T3 coliphage. Biochim Biophys Acta. 1970 May 21;209(1):24–33. doi: 10.1016/0005-2787(70)90657-x. [DOI] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. I. PURIFICATION AND SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1964 Jan;239:269–274. [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. II. KINETICS AND FEEDBACK CONTROL. J Biol Chem. 1964 Jan;239:275–284. [PubMed] [Google Scholar]
- Price A. R., Warner H. R. A structural gene for bacteriophage T4-induced deoxycytidine triphosphate-deoxyuridine triphosphage nucleotidohydrolase. Virology. 1968 Nov;36(3):523–526. doi: 10.1016/0042-6822(68)90183-9. [DOI] [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman I., Greenberg D. B. Ribonucleotide reductase genes of phage T4: map location of the thioredoxin gene nrdC. Virology. 1972 Jul;49(1):337–338. doi: 10.1016/s0042-6822(72)80040-0. [DOI] [PubMed] [Google Scholar]
- Yagil E., Rosner A. Effect of adenosine and deoxyadenosine on the incorporation and breakdown of thymidine in Escherichia coli K-12. J Bacteriol. 1970 Aug;103(2):417–421. doi: 10.1128/jb.103.2.417-421.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y. C., Dubovi E. J., Tessman I. Control of pyrimidine biosynthesis by phage T4: mutants unable to catalyze the reduction of cytidine diphosphate. Virology. 1969 Apr;37(4):615–623. doi: 10.1016/0042-6822(69)90279-7. [DOI] [PubMed] [Google Scholar]



