Figure 4.
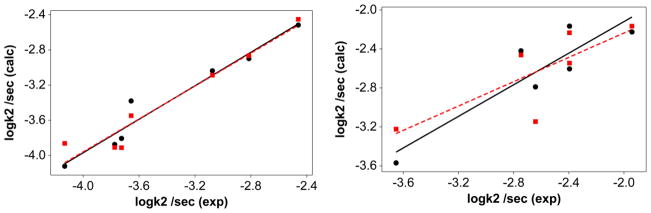
Plot of multicomponent fit of the rate constants at 298 K in acetonitrile for the solvolysis reactions with the experimental data. Left panel: compounds 2a–2g, circles with phosphine cone angles (eq 17) and square with phosphine oxide cone angles (eq 18). logk2 = −6.2 (±1.8) − 0.040 (± 0.26)pKa + 0.18 (±0.01)Θ + 0.38 (±0.11) Ear (R2=95) …eq 17; logk2 = −3.9 (±0.12) − 0.060 (± 0.030)pKa + 0.030(±0.040) (Θ-Θth)λ+0.31 (±0.21) Ear (R2=94)… eq 18.. Right panel: compounds 1a–1g, circles with phosphine cone angles (eq 19) and square with phosphine oxide cone angles (eq 20). logk2 = −10(±4.5) + 0.030(± 0.060)pKa + 0.060(±0.040) Θ+0.180 (±0.270) Ear (R2=81)…eq19; logk2 = −3.1 (±0.35) + 0.020 (± 0.080)pKa − 0.050 (±0.090) (Θ-Θth)λ+0.66 (±0.54) Ear (R2=62) …eq20.
