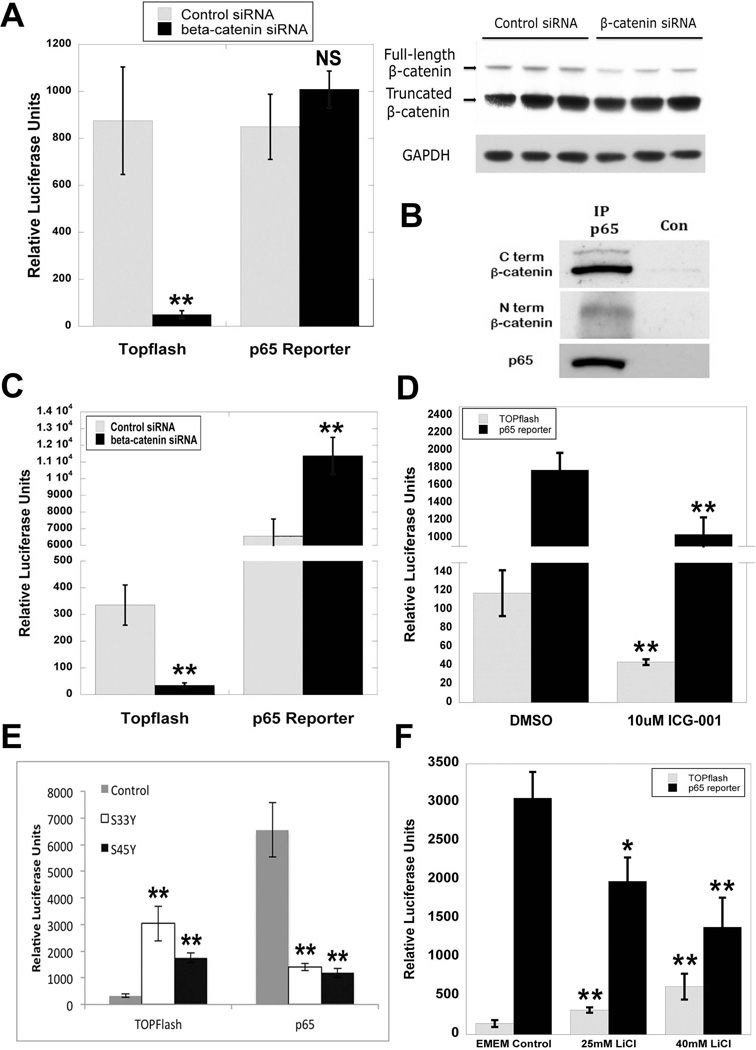
Figure 7. Modulation of β-catenin signaling in human hepatoma cell lines regulates p65 activity.
(A) HepG2 cells transfected with either control or β-catenin siRNA showed no significant differences in p65 reporter plasmid activity, despite a significant decrease in TOPflash activity (**P<0.01). WB demonstrates a decrease in full-length but not truncated β-catenin at 48 hours after siRNA treatment in HepG2 cells. GAPDH represents loading control.
(B) IP shows that p65 associates with both the full-length and truncated form of β-catenin in HepG2 cells.
(C) NF-κB activity as measured by p65 luciferase reporter is increased in Hep3B cells transfected with β-catenin siRNA (**P<0.01). Decrease in TOPflash reporter activity confirms knockdown of β-catenin (**P<0.01).
(D) p65 luciferase reporter activity is decreased in Hep3B cells transfected with β-catenin activity inhibitor ICG-001 (**P<0.01). Inhibition of β-catenin signaling is verified by a decrease in TOPflash reporter activity (**P<0.01).
(E) Transfection of Hep3B cells with mutant S33Y and S45Y β-catenin increases TOPflash activity but causes a decrease in p65 luciferase reporter activity as compared to control (**P<0.01).
(F) Treatment of Hep3B cells with escalating doses of LiCl increases TOPflash activity but causes a decrease in p65 luciferase reporter activity as compared to control (*P<0.05; **P<0.01).