Abstract
Many mammals can utilize social information to learn by observation of conspecifics (social learning). Social learning of fear is expected to be especially advantageous for survival. However, disruption of social development in early life can impair social cognition and might also be expected to disrupt social learning. Social isolation during a critical period of adolescence disrupts social development. The purpose of this study was to determine whether disruption of social development through post-weaning social isolation leads to impairments of social fear learning. Rats were reared in isolation or pair-housed from immediately post-weaning, for 3 weeks. Social fear learning in rats was acquired by observation of tone-footshock pairings administered to a conspecific. Isolation-reared rats displayed less conditioned freezing than pair-housed rats when tested the next day. This reduction of conditioned freezing was correlated with conspecific-oriented behaviors during conditioning, was measured despite similarities in demonstrator behaviors, and occurred despite a manipulation that equalized freezing during conditioning between the pair-housed and isolation-reared rats. The results could not be explained by abnormal sensitization to a repeated tone or deficits in freezing or direct fear conditioning. These results demonstrate that observational fear conditioning is impaired by social isolation, and provide a model to study impaired social affective learning. Impaired social cognition, manifested as inability to recognize or appropriately interpret social cues, is a symptom of several psychiatric disorders. Better understanding of the mechanisms of impaired social fear learning can lead to novel treatments for social cognition symptoms of psychiatric disorders.
Keywords: social, learning, observational, fear, conditioning, isolation-rearing
1. Introduction
Disruption of normal social interaction is a significant risk factor in a wide range of diseases [1, 2]. Furthermore, the ability to form appropriate social networks acts as a buffer against psychiatric disorders [3, 4]. Adolescence is a period of heightened social engagement [5, 6]. Reductions of peri-adolescent social interaction are associated with depression, impaired cognitive development, and behavioral social abnormalities [7–14]. Rats that undergo prolonged (>2 week) social deprivation that includes the forth and fifth weeks postnatal display a wide range of abnormal social behaviors, including increased aggression, abnormal interactions with novel rodents, and abnormal sexual behavior [6, 15–21]. Some of these behaviors share features of symptoms in several psychiatric disorders, such as social withdrawal, impaired social cognition, and inaccurate appraisal of others’ emotions. A better understanding of the factors that lead to disruptions of social interaction can provide new approaches for the treatment of these psychiatric symptoms.
A significant component of social development is the ability to learn by observation of peers, or social learning. Social learning has the obvious advantage of allowing individuals to gain knowledge of threats without direct experience. It is well established that non-human primates have the ability to learn by observation [22, 23]. Several studies now demonstrate that observation of distress in other rodents leads to heightened fear and anxiety in the observing rodent [24, 25], or behaviors to cope with the observed source of distress [26, 27]. This social transmission of fear state can influence subsequent fear learning [28]. Even social interaction with a recently fear-conditioned rodent imparts information to naïve rats and facilitates subsequent fear conditioning [25]. Furthermore, observation of an animal’s conditioned responses can lead to subsequent conditioned freezing by the observer [29, 30]. It has also been demonstrated that observation of a fear conditioning procedure can lead to conditioned freezing in the observer rodent [31]. Moreover, this ability is potentially influenced by social factors, as pro-social mice displaying better ability to learn by observation [31].
However, it is not known whether rodents with developmental social abnormalities are impaired in social fear learning. The purpose of this study was to test whether rodents that were socially isolated during post-weaning development display disruptions of observational learning. This was tested using an observational model of fear conditioning in rats that were isolation-reared for three continuous weeks after weaning.
2. Methods and Materials
All studies had prior approval of the Rosalind Franklin University Institutional Animal Care and Use Committee, and complied with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
2.1 Animals
Male Sprague Dawley rats from Harlan Laboratories (Madison, Wisconsin) were used for these studies. Rats arrived at the Rosalind Franklin University vivarium at 20–21 days postnatal (rats were weaned at 18–19 days postnatal). The rats were provided water and food ad libitum. The housing rooms were set to a 12h/12h light-dark cycle. Temperature was maintained between 64–79 degrees Fahrenheit, and the humidity was maintained between 30–70%. Upon arrival, all animals were either pair-housed or isolation-housed. Rats were further subdivided into those that would undergo direct fear conditioning (demonstrators) and those that would observe fear conditioning (observers). Demonstrators were housed with other demonstrators, and observers were housed with other observers (when pair-housed). The assignments were random. Rats were handled daily for three days prior to fear conditioning.
2.2 Fear Conditioning
2.2.1. Apparatus
Auditory cued fear conditioning and fear testing were performed in two distinct behavioral chambers. Chambers were kept inside sound-attenuating cabinets that were cleaned with distinct odorants (1% acetic acid or 10% Simple Green). Texture, color and pattern of the walls and flooring were different between the two chambers. Each cabinet was affixed with an IR-sensitive digital camera (Fire-i, Unibrain, San Ramon, CA), infrared lighting, and white lighting. There was a fan in each cabinet that provided airflow and 60–70 dB of ambient noise. Tones were delivered through speakers inside the cabinets (2000 Hz, 80–85 dB). Footshocks were delivered through a grid floor in the chambers. A footshock intensity was chosen that induced a strong paw withdrawal response and occasional vocalization from the demonstrator rat (0.5 mA or 0.8 mA; different configuration of chambers required different level of footshock to induce behavioral response). In all experiments, chamber use was alternated and counterbalanced between groups. The footshock and tone were generated in a reproducible manner (programmable sound generator 46000-164, Ugo Basile, Italy), and software-controlled (AnyMaze software, Wood Dale, IL). The video feed was collected on a computer hard drive as experiments were conducted. Freezing was measured using AnyMaze software based on detection of changes in pixel luminosity of the videos. Video detection was enhanced using an IR-sensitive camera and an IR light source kept close to the chambers. Detection thresholds were set based upon >90% convergence with a manual rater.
Prior to observational fear conditioning, chambers were modified to accommodate two rats. The chamber was divided into two equal sides, separated by a plexiglass mesh barrier. The only difference between the two sides was a thin piece of plexiglass placed above the footshock grid in one side, while the footshock grid remained exposed in the other side.
2.2.2. Fear conditioning procedure
All experiments were performed during the light phase of the light-dark cycle. When pair-housed rats were utilized, cage mates always received the same behavioral treatment. Experiments with isolation-reared and pair-housed rats were interleaved such that experiments on both groups were performed over the same period of time. Rats were taken from their home cages and placed in transport cages, one rat to each transport cage. The transport cages were taken to the procedure room, where rats were placed in the chambers. One rat was placed on either side of the chamber. The demonstrator rat that was placed on the footshock-exposed side was always a pair-housed rat. An isolation-reared observer rat or a pair-housed observer rat, was placed in the other side. A two minute habituation period began immediately following placement in chambers. Following habituation, eight successive trials of tone-foot shock pairings were performed. During each trial, a 10 s tone co-terminated with a 1 s footshock. The intertrial interval was 80 s. Rats were returned to their home cages after fear conditioning. The number of pellets defecated and the presence or absence of a urine puddle was noted.
One day following fear conditioning, fear testing was performed. Single rats were placed into a novel chamber, with just one rat in each chamber. Rats explored the novel chamber for a two minute habituation period. Following this habituation, the tone (2000 Hz, 20 s) was presented, with an inter-trial interval of 80 s.
Other behavioral measures during conditioning were obtained. These include the total time proximal to the barrier that separated the demonstrator and observer, the total time oriented towards the demonstrator, and the total distance traveled during conditioning. To determine the time proximal to the barrier, the observer’s portion of the chamber was divided into 3 equal longitudinal rectangles. One rectangle was proximal to the barrier. The time proximal to the barrier was quantified as the average amount of time during each trial that the observer rat’s head was in this proximal third of the chamber. To measure the time oriented towards the demonstrator, a rater tracked the position of the rodent’s head. The head was judged to be oriented towards the conspecific when its nose was perpendicular to the barrier (pointed at the barrier) ± 30 degrees to either side. To aid determination of head orientation, a template was made (60 degree angle) that was held perpendicular to the barrier on the video monitor during analysis. For these measures, the rater was blind to the animal group. Head orientation towards conspecifics was measured for each trial and averaged across trials. The total distance traveled during each trial was tracked by software (AnyMaze).
2.3. Footshock sensitivity
The response of rats to footshock was measured. This was quantified on an ordinal scale (0= no observable response, 1=flinch, 2=forepaw withdrawal, 3=scramble, 4=run in circle, 5=attempt to jump out of chamber). This assay was used to determine if there was an effect of isolation on sensitivity to footshock and to measure the behavior of demonstrators during conditioning. In a group of rats, footshock threshold was quantified by increasing footshock stimulation intensity until flinching was observed in response to the footshock.
2.4. Analysis
The total freezing time during trials (over the entire 80 s intertrial interval) was measured and converted to percent of time freezing [(freezing time ÷ trial time) × 100]. The percent of time freezing was compared across groups with two-way ANOVAs (GraphPad Prism, La Jolla, CA). Significance was set at p<0.05. If a significant result was obtained, groups were further compared with one-way ANOVAs or Newman-Keuls multiple comparisons test. Rats that displayed more than 20 s freezing during habituation or no freezing during conditioning were excluded from analysis. This included 2 rats that were directly conditioned (1 rat that displayed >20 s freezing during habituation, and 1 rat that displayed no freezing during testing) and 3 rats that observed conditioning (2 rats that displayed >20 s freezing during habituation, and 1 rat that displayed no freezing during testing). The time proximal to the barrier, the time oriented towards the barrier, and the total distance traveled was analyzed using two-tailed unpaired t-tests. The number of pellets was quantified and compared with non-parametric analysis (Mann-Whitney U). The proportion of rats that urinated during conditioning was compared with Chi-square analysis.
3. Results
3.1. Conditioning by observation
During conditioning, a demonstrator rat underwent a fear conditioning procedure that paired eight tones with co-terminating footshocks (Methods section 2.2). In the same behavioral chamber, but separated by a plexiglass mesh barrier, an observer rat was present during the conditioning procedure. This observer did not experience footshocks because its side of the chamber included a thin plexiglass cover over the footshock grid. No portion of the observer rat could make contact with the footshock grid. The demonstrator rats displayed behavioral responses to the footshock itself (flinching, front paw withdrawal, and occasional vocalizations) and increased freezing in response to the tone over the course of the fear conditioning protocol (Fig 1A; repeated measures ANOVA, p<0.0001, F(8,269)=157.1, n=30 rats). Rats that observed the fear conditioning also displayed increased freezing over the course of the conditioning (Fig 1B; two-way repeated measures ANOVA, Trials x Housing, p<0.0001, F(8,224)=34.5, significant main effect of trial).
Figure 1. Isolation-reared observer rats display less conditioned freezing after observational fear conditioning than pair-housed rats.
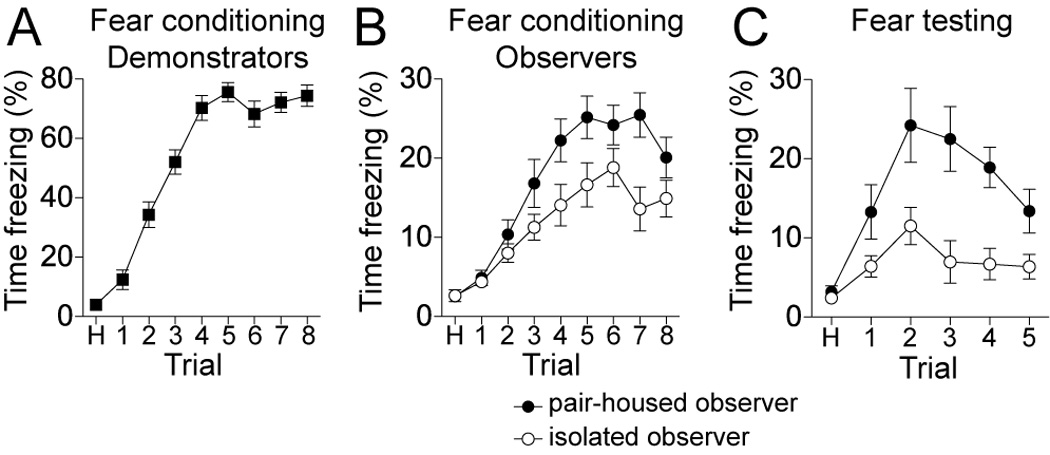
Fear conditioning was performed in a two-compartment chamber that allowed one rat to observe another rat undergo repeated pairings of an auditory cue with a co-terminating footshock. A) The rats that directly experienced the footshock displayed increasing freezing over the course of the 8 conditioning trials. B) Rats that observed the cue-footshock pairings also displayed increased freezing over repeated trials. However, rats that underwent three weeks of post-weaning social isolation displayed significantly less freezing during the conditioning. Note the different y-axis scale in A and B. C) During testing one day later, the auditory cue was presented alone to individual rats. Isolation-reared rats displayed significantly less conditioned freezing during testing compared to pair-housed rats. Black represents pair-housed animals, white represents isolation-reared animals.
3.2. Social isolation causes deficits in conditioning by observation
Isolation-reared rats displayed significantly less freezing during observational conditioning than pair-housed rats (Fig 1B; two-way repeated measures ANOVA, Trials × Housing, p=0.013, F(1,224)=7.01, significant main effect of housing group). The day after observational conditioning, conditioned freezing to the tone by the observer rats was tested in a novel context. These observer rats demonstrated significant freezing to the conditioned tone (Fig 1C; one-way repeated measures ANOVA, compared freezing during test day habituation p<0.0001, F(5,29)=12.3, all trials significantly different (p<0.05) Newman-Keuls multiple comparison test, and compared to freezing during first tone presentation on conditioning day p<0.0001, F(5,29)=12.4, all trials significantly different (p<0.05) than freezing to the first tone on the conditioning day). However, rats that were isolation-reared displayed significantly less conditioned freezing when tested for freezing to the conditioned tone (Fig 1C; two-way repeated measures ANOVA, Trials × Housing, p=0.0026, F(1,140)=10.9, significant main effect of housing group; peak freezing controls: 24.2 ± 4.7% freezing, isolation-reared 11.5 ± 2.4% freezing, p=0.022, t=2.43, n=15 rats/group). During fear testing, rats displayed less freezing during the first tone presentation than in subsequent presentations (e.g. Fig 1C). This may be due to a negative association between conditioned freezing and exploration of a novel environment in some rat strains. This effect is not observed, and may have been mitigated, if a longer (>3 min; e.g. Maren et al, 1998) habituation period was utilized prior to testing in the novel context.
3.3. Differences in acquisition do not fully account for effects of social isolation
The deficit of conditioned freezing on the testing day in isolation-reared rats may have been due to deficient acquisition of observational conditioning. Consistent with this, isolation-reared rats displayed significantly less freezing during observational conditioning (above, section 3.2). To test whether reduced acquisition of observational conditioning may underlie reduced conditioned freezing on the testing day, a separate group of pair-housed rats underwent observational fear conditioning using only 3 trials, to attain a much lower degree of freezing during conditioning (Fig 2A; peak freezing 18.0 ± 1.7%, n=9 rats) that was similar to the isolation-reared rats (peak freezing 18.8 ± 2.4% in the 8 trial conditioning, n.s., p=0.81, t=0.24, two-tailed t-test). However, when tested the following day for conditioned freezing to the tone, these paired-housed rats that were conditioned with 3 trials still displayed significantly greater conditioned freezing than the isolation-reared rats that were conditioned with 8 trials (Fig 2B; two-way repeated measures ANOVA, Conditioning protocol × Housing, p=0.017, F(1,110)=6.64, significant mail effect of housing group). Thus, despite similar amounts of freezing on the observational conditioning day, social isolation impaired conditioned freezing on the testing day in observer rats.
Figure 2. Isolation-rearing impairs observational learning even when freezing during observational conditioning is equivalent between isolation-reared and pair-housed groups.
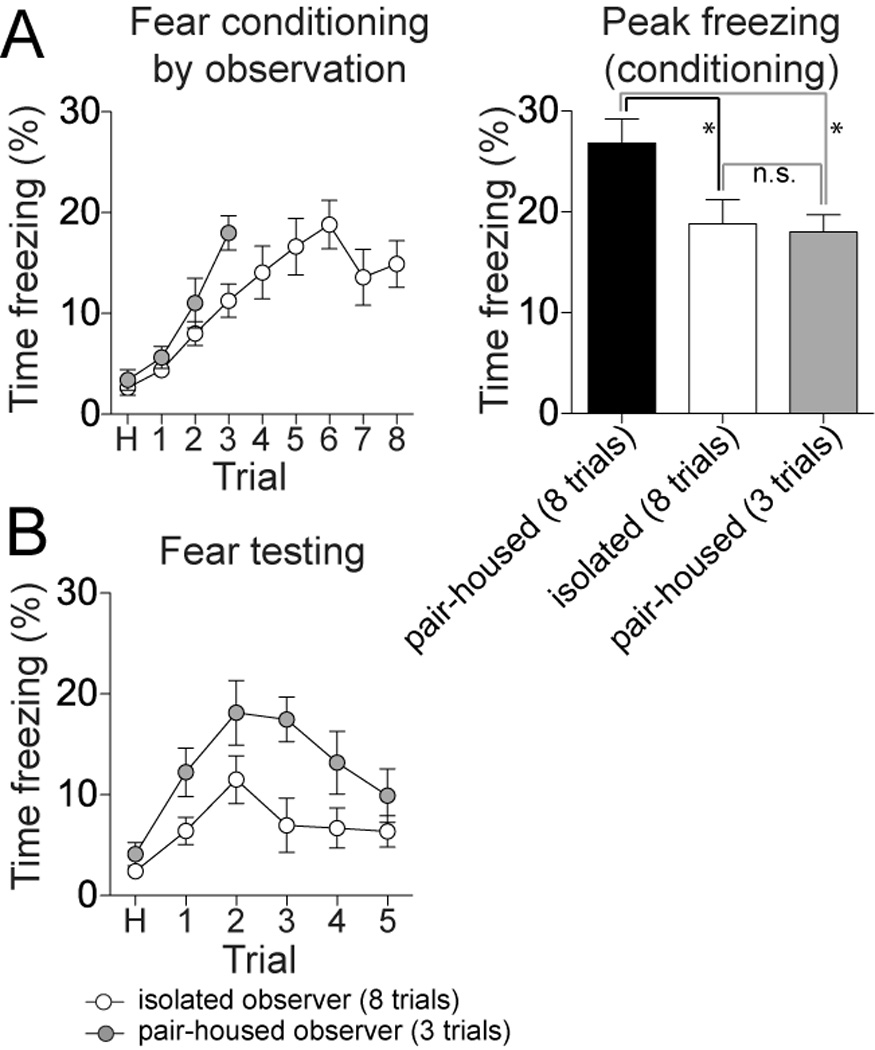
To achieve equivalent levels of freezing during observational fear conditioning, pair-housed rats were administered a truncated (3 trials of cue + footshock) observational fear conditioning protocol. A) The truncated observational fear conditioning led to a similar level of freezing during conditioning in pair-housed rats as the full (8 trial) conditioning in isolation-reared rats (left). There was no significant difference in the peak levels of freezing achieved between the full conditioning protocol in isolation-reared rats and the truncated protocol in pair-housed rats (right). B) Despite similar levels of freezing during observational fear conditioning, isolation-reared rats conditioned with the full (8 trial) protocol still displayed significantly less conditioned freezing during retrieval than the pair-housed rats conditioned with the truncated (3 trial) protocol. Black represents pair-housed rats that were conditioned with the full 8 trial protocol, white represents isolation-reared rats that were conditioned with the full 8 trial protocol, and grey represents pair-housed rats that were conditioned with the truncated 3 trial protocol. * represents p<0.05 in Newman-Keuls multiple comparisons test after a one-way ANOVA.
3.4. Correlations with conditioned freezing
To gain more insight into factors that contribute to the effects of social isolation on observational conditioning, correlations were calculated between average conditioned freezing on the testing day and several factors during conditioning. In pair-housed control rats there was a significant correlation between the time spent proximal to the barrier on the conditioning day and the conditioned freezing on the testing day (Fig 3A; R square = 0.67, p=0.0002), but not in isolation-reared rats (R square = 0.13, p=0.19). There was no significant difference in the amount of time spent near the barrier between pair-housed controls and isolation-reared rats (Fig 3A; pair-housed 15.5 ± 2.9 s, isolation-reared 13.6 ± 2.1 s, p=0.59, t=0.54, df=28, two-tailed unpaired t-test). In pair-housed controls there was also a significant correlation between the time oriented towards the demonstrator rat during conditioning and the time freezing during testing (Fig 3B; R square = 0.50, p=0.0031), but no such correlation existed in isolation-reared rats (R square = 0.18, p=0.12). The amount of time spent oriented towards the demonstrator was significantly less in isolation-reared rats (Fig 3B; pair-housed 20.1 ± 2.2 s, isolation-reared 12.2 ± 1.4 s, p=0.005, t=3.04, df=28, two-tailed unpaired t-test).
Figure 3. Behavior of pair-housed, but not isolation-reared, rats during observational conditioning was correlated with conditioned freezing.
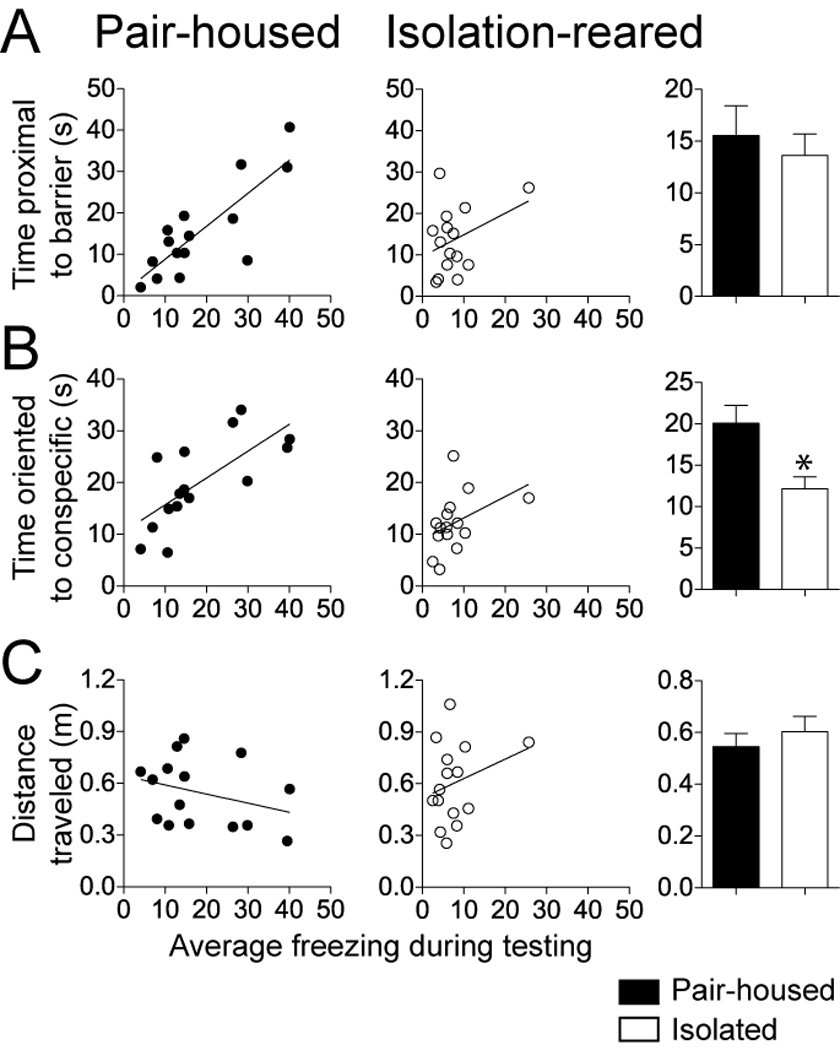
A) There was a significant correlation between the time spent close to the barrier that separated the demonstrator and observer rats during conditioning and the conditioned freezing displayed by the observer rat during testing. This correlation was observed in pair-housed but not isolation reared rats. There was no significant difference between groups in the average amount of time spent close to the barrier. B) There was a significant correlation between the amount of time that the observer rat’s head was oriented towards the demonstrator during conditioning and the amount of conditioned freezing displayed on the testing day. This correlation was observed for the pair-housed but not the isolation-reared rats. Pair-housed rats also displayed significantly greater average amount of time oriented towards the demonstrator rat during conditioning. C) There was no significant correlation between the distance traveled during conditioning and the conditioned freezing displayed during testing. Pair-housed and isolation-reared rats displayed a similar distance traveled during conditioning. Black represents pair-housed observer, white represents isolation-reared observer. * indicates p<0.05 two-tailed unpaired t-test.
There were no correlations between the total distance traveled during the conditioning and conditioned freezing on the test day for either group (Fig 3C; pair-housed R square = 0.10, p=0.25; isolation-reared R square = 0.08, p=0.32), and no significant differences in the distance traveled during conditioning between groups (pair-housed 0.55 ± 0.05 m, isolation-reared 0.60 ± 0.06 m; p=0.47, t=0.73, df=28, two-tailed unpaired t-test). A significant difference in the orientation towards the demonstrator rat, and its significant correlation to the eventual conditioned response in control but not in isolation-reared rats may be indicative of the deficient social cognition in isolation-reared rats.
3.5. Characteristics of demonstrators
All demonstrators were pair-housed. Nevertheless, the demonstrators may have behaved differently for the pair-housed and isolation-reared observers and thereby influenced the behavior of the observers. To test this, the freezing of the demonstrators was measured during fear conditioning and testing. There was no significant difference between demonstrators for pair-housed and demonstrators for isolation-reared rats in freezing during fear conditioning (Fig 4A; two-way repeated measures ANOVA, Trials × Observer housing, p=0.92, F(1,224) = 0.010, no significant effect of observer group on demonstrator freezing), nor demonstrator freezing during fear testing (Fig 4B; two-way repeated measures ANOVA, Trials × Observer housing, p=0.77, F(1,224) = 0.09, no significant effect of observer group on demonstrator freezing). Furthermore, the demonstrator groups displayed no significant difference from each other in their behavioral response to footshock over the course of conditioning (Fig 4C; two-way repeated measures ANOVA, Trials × Observer housing, p=0.39, F(1,224)=0.75). Nor was there a significant difference in the number of pellets defecated by the demonstrators for pair-housed or isolation-reared rats during conditioning (Fig 4D; demonstrator for control 3.9 ± 0.5 pellets, demonstrator for isolation-reared 4.1 ± 0.7 pellets, p=0.98, Mann-Whitney U=111.5), nor proportion of demonstrators that urinated during the conditioning (Fig 4E; demonstrator for pair-housed 12/15 rats, demonstrator for isolation-reared 13/15 rats, p=0.62, Chi-square). Therefore, differences in observational conditioning in pair-housed and isolation-reared observers are unlikely due to differences in demonstrator behavior.
Figure 4. Demonstrators for pair-housed and isolation-reared rats displayed no significant differences in their behaviors.
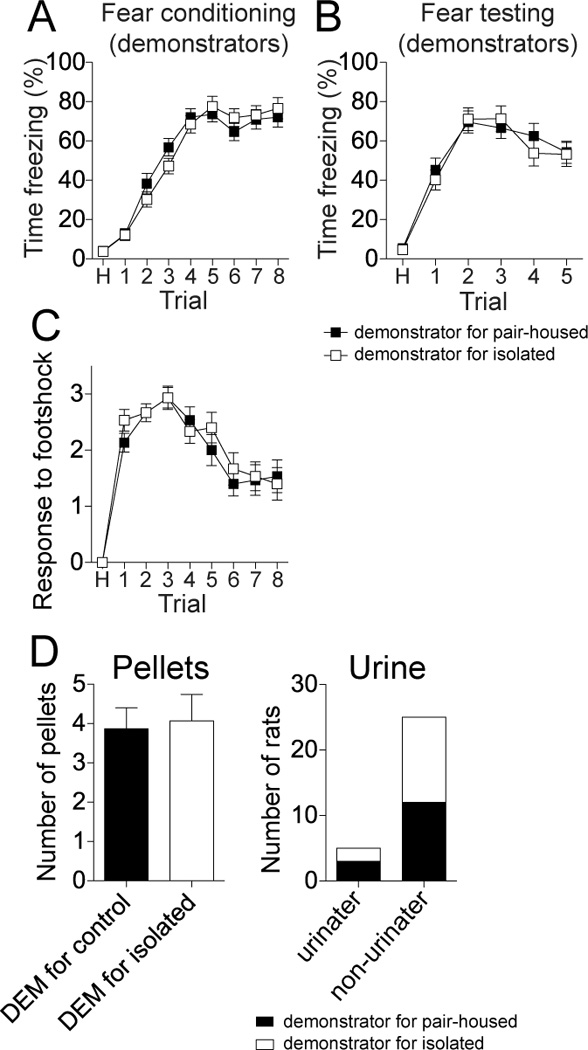
A) Rats that were demonstrators for pair-housed rats displayed a similar degree of freezing during conditioning as demonstrators for isolation-reared rats. B) As further indication of their similarity, rats that were demonstrators for pair-housed rats displayed a similar degree of conditioned freezing during testing as demonstrators for isolation-reared rats. C) The behavioral response to footshock during fear conditioning was similar between both groups of demonstrators across the conditioning trials. D) There was no significant difference in the number of pellets defecated by the demonstrators for paired-housed and demonstrators for isolation-reared rats during fear conditioning (left). There was no significant difference in the proportion of demonstrators that urinated during fear conditioning (right). Black represents demonstrator for pair-housed rat, white represents demonstrator for isolation-reared rat.
3.6. No sensitization to repeated auditory cue
The measured freezing by the observers in response to the tone may possibly be unrelated to social learning, and perhaps only represent a response to repeated presentation of a tone. To test this, a separate group of rats was presented with tones in the same manner as the observational conditioning procedure, but with no footshock. These rats were then tested for their freezing response to the tone the following day. The freezing response to the tone was minimal for pair-housed and isolation-reared rats (Fig 4), and was not significantly different from each other during the mock conditioning (Fig 4A; two-way repeated measures ANOVA, Trials × Housing, p=0.41, F(1,112)=0.71, n=8 rats/group, no significant effect of isolation on response to repeated tone presentation), nor during the testing the next day (Fig 4B; two-way repeated measures ANOVA, Trials × Housing, p=0.27, F(1,70)=1.31, no significant effect of isolation on response to repeated tone presentation). Therefore, abnormal sensitization to the tone is unlikely to underlie observational conditioning, or the group differences in these experiments.
3.7. Social isolation does not impair direct fear conditioning
Another alternative explanation is that social isolation impairs learning, and the impairment of learning by observation is an extension of this impairment. To test this, traditional fear conditioning was performed in a separate group of pair-housed and isolation-reared rats. In these experiments the pair-housed or isolation-reared rat was placed alone in the fear conditioning and testing chambers. There was no significant impairment of freezing during fear conditioning (Fig 5A; no significant effect in two-way repeated measures ANOVA, Trials × Housing, p=0.055, F(1,144)=4.22, n=10 rats/group) or during fear testing the next day (Fig 5B; no significant effect in two-way repeated measures ANOVA, Trials × Housing, p=0.21, F(1,90)=1.72) in isolation-reared rats. In fact, there was a trend towards increased freezing in the isolation-reared rats upon direct fear conditioning. These data also indicate that isolation-reared rats do not have a deficit in the ability to freeze. In addition, in a separate group of rats, there was no significant effect of isolation-rearing on the response to footshocks, measured as the threshold to flinch in response to footshock (p=0.81, two-tailed t-test, t=0.24, 10 rats/group), or as the behavioral response to increasing footshock intensity (no significant effect of isolation, two-way repeated measures ANOVA, Footshock intensity × Housing, p=0.11, F(1,60)=3.04, n=8 rats/group). Therefore, it is unlikely that deficits in associative fear learning underlie the impaired observational learning in isolation-reared rats. Furthermore, it also is unlikely that differences in response to footshock mask differences in fear conditioning between pair-housed and isolation-reared rats.
Figure 5. Isolation-reared rats do not display sensitized responses to auditory cues alone.
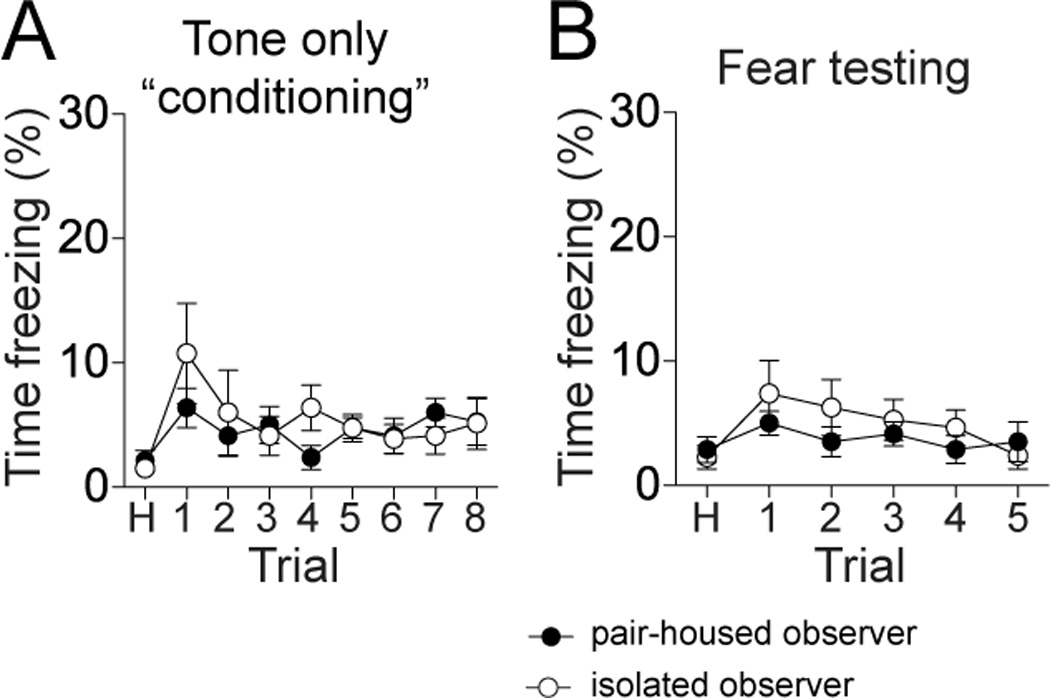
A) Rats were presented with the same observational fear conditioning procedure in the absence of footshock. Observer rats did not display increased freezing over the course of auditory tone presentation trials. B) When tested after one day, neither pair-housed nor isolation-reared observer rats displayed evidence of increased responding to the tone. Black represents pair-housed observer, white represents isolation-reared observer.
4. Discussion
Social learning by observation of conspecifics is advantageous, but likely relies on robust social cognition. If social learning of conditioned freezing relies on normal social cognition, then a treatment that disrupts social development is expected to disrupt social learning. This study demonstrates that social isolation during rearing leads to an impairment of social transmission of conditioned fear.
The ability of rodents to learn by observation of other rodents has been demonstrated in numerous situations, including transmission of food preference and fear learning [24, 25, 27, 28, 30–34]. The results here confirm social transmission of fear by observation of a conspecific during fear conditioning. While the degree of freezing measured after observational fear conditioning was less than directly fear conditioning, it was still significant and behaviorally impactful. This observational fear conditioning was not related to sensitization of responses to an auditory cue, as repeated tones in the absence of footshock did not lead to increased freezing. In pair-housed control rats the degree of observational conditioned freezing was correlated with the time the observer rat was oriented towards the demonstrator and in proximity to the demonstrators’ side of the chamber. This implies that social information passed by attending to the demonstrator contributes to observational conditioning.
This transit of social information was impaired in rats that experienced post-weaning social isolation. Previous studies have demonstrated disruption of social learning of food preference upon pre-weaning maternal and sibling deprivation (e.g. [35, 36]). Prolonged (>2 week) post-weaning social isolation reduces contextual learning [37], impairs rule learning [38], and causes deficits in novel object recognition [39–41], indicative of cognitive deficits. At least one study indicated a deficit of fear conditioning after social isolation in mice [42]. However, the impairment in observational conditioning after social isolation in this study was not attributable to deficits in ability to learn, as isolation-reared rats learned fear conditioning that was directly experienced. Consistent with the direction of these findings, a previous study demonstrated faster acquisition of associative learning in isolation-reared rats [43]. The impaired observational learning in isolation-reared rats was also not explained by differences in demonstrator behavior, differences in the level of freezing during acquisition, or ability to freeze. However, it may be related to the decreased orientation of the isolation-reared observer rats towards the demonstrators. Consistent with decreased processing of social cues in isolation-reared rats, social isolation disrupts social recognition in rodents [44]. The exact nature of the deficit in social learning in this study depends upon the nature of the cues utilized by the observing rodent, and was not studied here. In other studies, odor and vocalization contribute to social transmission of fear [45–47] and contribute to social modulation of other behaviors in rodents [48, 49]. The resulting deficit in fear conditioning appears to include diminished social fear learning, as well as reduced conditioned freezing to a socially-learned cue. This reduced conditioned freezing may include components of consolidation, recall or expression of the socially learned cue.
There are a number of possible routes to conditioned freezing after observational fear conditioning. It is currently not known whether the observing rat forms an association between the tone and an aversive outcome to a conspecific, an association between the tone and a transmitted social cue that itself may be an aversive unconditioned stimulus (such as an ultrasonic vocalization), whether the association is between the tone and a previously conditioned stimulus (such as a previous association between an observed rat’s unconditioned response and an aversive event occurring to the observer), or whether the rat is displaying “empathy” towards the affective behaviors of the demonstrating rat.
Several studies emphasize an important role for the amygdala in observational fear. In humans, the amygdala is activated in response to social stimuli, such as faces and facial expressions [50–52] and during observational fear conditioning [53]. Lesions to the amygdala in humans lead to deficits in recognition of facial expressions [54], and diminished orientation towards socially relevant cues on the face [55]. In primates, lesions of the amygdala result in abnormal social behavior (Kluver and Bucy, 1939), and amygdala neurons are responsive to social stimuli [56–58]. In rodents, social transmission of fearful state is associated with increased expression of c-Fos in the amygdala [28], and amygdala neurons are activated during social interaction [59]. Social isolation may disrupt a component of the amygdala circuit involved in observational conditioning. In support of this, social isolation leads to morphological changes in the medial amygdala [20], which plays a fundamental role in rodent social behavior [60, 61]. Social isolation also leads to abnormalities of the dopamine system in the amygdala [62–64], which plays a significant role in associative fear learning [65–67]. Furthermore, early life social deprivation leads to abnormal amygdala activity in humans [68, 69].
The experiments in this study examine an interface between social cognition and affect. There are several psychiatric disorders that display aspects of impaired social cognition that contributes to abnormal affect, such as autism and schizophrenia. A component of these symptoms may include inability to appreciate or express emotion and impaired social interactions. A better understanding of the substrates of social components of mood and emotion can provide understanding of the neurobiology of these symptoms, and perhaps lead to novel therapeutic targets for alleviation of these symptoms.
Figure 6. Social isolation does not impair direct fear conditioning.
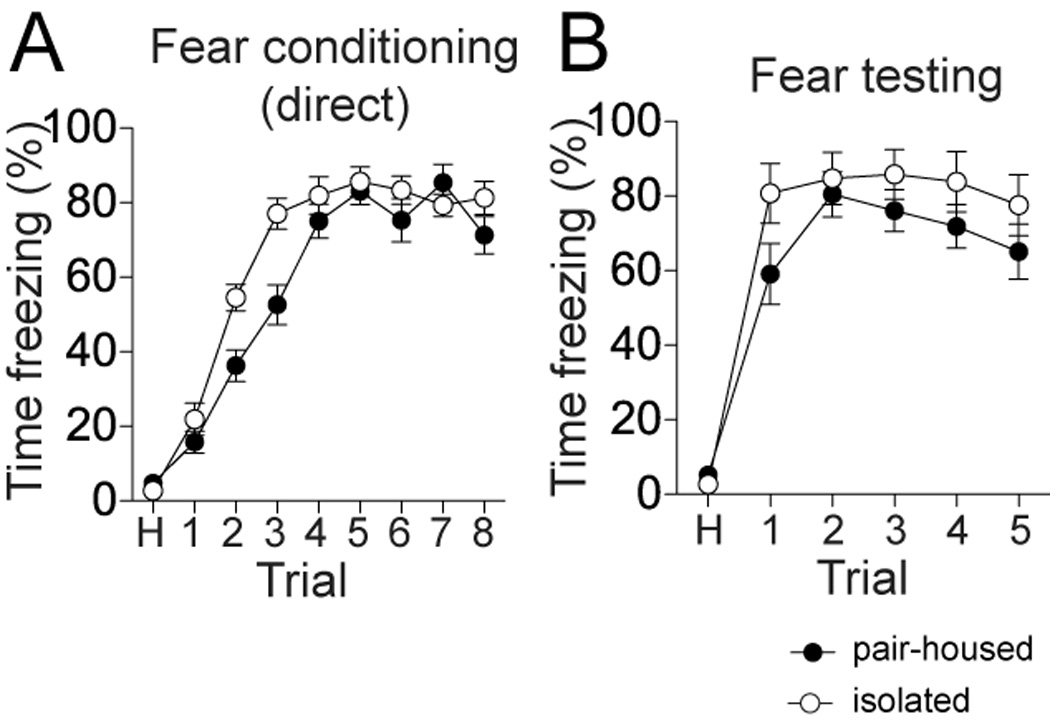
A) When exposed directly to auditory cue-footshock pairing, pair-housed and isolation reared rats displayed similar increases in freezing over the course of conditioning trials. B) When tested after one day, pair-housed and isolation-reared rats displayed similar levels of conditioned freezing. Black represents pair-housed observer, white represents isolation-reared observer.
Highlights.
Freezing response is acquired by observation of tone-shock pairings to conspecific
Correlated with orientation towards conspecific during conditioning
Observational fear conditioning is impaired by rearing in social isolation
Impairment not due to impaired freezing or associative learning disability
Understanding cause may lead to new treatment approach for socio-cognitive symptoms
Acknowledgements
The authors wish to thank Mallika Padival for technical help and Dr. Janice Urban for helpful discussion. This research was supported by NIH grant MH084970.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000316. e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 3.Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychol Aging. 2006;21:140–151. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- 4.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- 5.Larson R, Richards MH. Daily companionship in late childhood and early adolescence: changing developmental contexts. Child Dev. 1991;62:284–300. doi: 10.1111/j.1467-8624.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 6.Meaney MJ, Stewart J. A descriptive study of social development in the rat (Rattus norvegicus) Anim Behav. 1981;29:34–45. [Google Scholar]
- 7.Colvert E, Rutter M, Beckett C, Castle J, Groothues C, Hawkins A, Kreppner J, O'Connor TG, Stevens S, Sonuga-Barke EJ. Emotional difficulties in early adolescence following severe early deprivation: findings from the English and Romanian adoptees study. Dev Psychopathol. 2008;20:547–567. doi: 10.1017/S0954579408000278. [DOI] [PubMed] [Google Scholar]
- 8.Castle J, Groothues C, Bredenkamp D, Beckett C, O'Connor T, Rutter M. Effects of qualities of early institutional care on cognitive attainment. E.R.A. Study Team. English and Romanian Adoptees. Am J Orthopsychiatry. 1999;69:424–437. doi: 10.1037/h0080391. [DOI] [PubMed] [Google Scholar]
- 9.Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 10.Hall-Lande JA, Eisenberg ME, Christenson SL, Neumark-Sztainer D. Social isolation, psychological health, and protective factors in adolescence. Adolescence. 2007;42:265–286. [PubMed] [Google Scholar]
- 11.Jacobson S, Fasman J, DiMascio A. Deprivation in the childhood of depressed women. J Nerv Ment Dis. 1975;160:5–14. doi: 10.1097/00005053-197501000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson KC, Rowe DC. Genetic and environmental influences on the relationships between family connectedness, school connectedness, and adolescent depressed mood: sex differences. Dev Psychol. 1999;35:926–939. doi: 10.1037//0012-1649.35.4.926. [DOI] [PubMed] [Google Scholar]
- 13.Klineberg E, Clark C, Bhui KS, Haines MM, Viner RM, Head J, Woodley-Jones D, Stansfeld SA. Social support, ethnicity and mental health in adolescents. Soc Psychiatry Psychiatr Epidemiol. 2006;41:755–760. doi: 10.1007/s00127-006-0093-8. [DOI] [PubMed] [Google Scholar]
- 14.Geckova A, van Dijk JP, Stewart R, Groothoff JW, Post D. Influence of social support on health among gender and socio-economic groups of adolescents. Eur J Public Health. 2003;13:44–50. doi: 10.1093/eurpub/13.1.44. [DOI] [PubMed] [Google Scholar]
- 15.Toth M, Tulogdi A, Biro L, Soros P, Mikics E, Haller J. The neural background of hyper-emotional aggression induced by post-weaning social isolation. Behav Brain Res. 2012;233:120–129. doi: 10.1016/j.bbr.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 16.van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol. 1999;34:129–138. [PubMed] [Google Scholar]
- 17.Hol T, Van den Berg CL, Van Ree JM, Spruijt BM. Isolation during the play period in infancy decreases adult social interactions in rats. Behav Brain Res. 1999;100:91–97. doi: 10.1016/s0166-4328(98)00116-8. [DOI] [PubMed] [Google Scholar]
- 18.Von Frijtag JC, Schot M, van den Bos R, Spruijt BM. Individual housing during the play period results in changed responses to and consequences of a psychosocial stress situation in rats. Dev Psychobiol. 2002;41:58–69. doi: 10.1002/dev.10057. [DOI] [PubMed] [Google Scholar]
- 19.Gerall HD, Ward IL, Gerall AA. Disruption of the male rat's sexual behaviour induced by social isolation. Anim Behav. 1967;15:54–58. doi: 10.1016/s0003-3472(67)80010-1. [DOI] [PubMed] [Google Scholar]
- 20.Cooke BM, Chowanadisai W, Breedlove SM. Post-weaning social isolation of male rats reduces the volume of the medial amygdala and leads to deficits in adult sexual behavior. Behav Brain Res. 2000;117:107–113. doi: 10.1016/s0166-4328(00)00301-6. [DOI] [PubMed] [Google Scholar]
- 21.Agis-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci U S A. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook M, Mineka S. Selective associations in the observational conditioning of fear in rhesus monkeys. J Exp Psychol Anim Behav Process. 1990;16:372–389. [PubMed] [Google Scholar]
- 23.Mineka S, Davidson M, Cook M, Keir R. Observational conditioning of snake fear in rhesus monkeys. J Abnorm Psychol. 1984;93:355–372. doi: 10.1037//0021-843x.93.4.355. [DOI] [PubMed] [Google Scholar]
- 24.Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knapska E, Nikolaev E, Boguszewski P, Walasek G, Blaszczyk J, Kaczmarek L, Werka T. Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proc Natl Acad Sci U S A. 2006;103:3858–3862. doi: 10.1073/pnas.0511302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kavaliers M, Colwell DD, Choleris E. NMDA-mediated social learning of fear-induced conditioned analgesia to biting flies. Neuroreport. 2001;12:663–667. doi: 10.1097/00001756-200103260-00009. [DOI] [PubMed] [Google Scholar]
- 27.Kavaliers M, Choleris E, Colwell DD. Learning from others to cope with biting flies: social learning of fear-induced conditioned analgesia and active avoidance. Behav Neurosci. 2001;115:661–674. [PubMed] [Google Scholar]
- 28.Knapska E, Mikosz M, Werka T, Maren S. Social modulation of learning in rats. Learn Mem. 2010;17:35–42. doi: 10.1101/lm.1670910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzman YF, Tronson NC, Guedea A, Huh KH, Gao C, Radulovic J. Social modeling of conditioned fear in mice by non-fearful conspecifics. Behav Brain Res. 2009;201:173–178. doi: 10.1016/j.bbr.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruchey AK, Jones CE, Monfils MH. Fear conditioning by-proxy: social transmission of fear during memory retrieval. Behav Brain Res. 2010;214:80–84. doi: 10.1016/j.bbr.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS One. 2009;4:e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts M, Shapiro M. NMDA receptor antagonists impair memory for nonspatial, socially transmitted food preference. Behav Neurosci. 2002;116:1059–1069. doi: 10.1037/0735-7044.116.6.1059. [DOI] [PubMed] [Google Scholar]
- 33.Galef BG, Jr, Kennett DJ. Different mechanisms for social transmission of diet preference in rat pups of different ages. Dev Psychobiol. 1987;20:209–215. doi: 10.1002/dev.420200209. [DOI] [PubMed] [Google Scholar]
- 34.Zentall TR, Levine JM. Observational learning and social facilitation in the rat. Science. 1972;178:1220–1221. doi: 10.1126/science.178.4066.1220. [DOI] [PubMed] [Google Scholar]
- 35.Melo AI, Lovic V, Gonzalez A, Madden M, Sinopoli K, Fleming AS. Maternal and littermate deprivation disrupts maternal behavior and social-learning of food preference in adulthood: tactile stimulation, nest odor, and social rearing prevent these effects. Dev Psychobiol. 2006;48:209–219. doi: 10.1002/dev.20130. [DOI] [PubMed] [Google Scholar]
- 36.Levy F, Melo AI, Galef BG, Jr, Madden M, Fleming AS. Complete maternal deprivation affects social, but not spatial, learning in adult rats. Dev Psychobiol. 2003;43:177–191. doi: 10.1002/dev.10131. [DOI] [PubMed] [Google Scholar]
- 37.Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Jones GH, Marsden CA, Robbins TW. Behavioural rigidity and rule-learning deficits following isolation-rearing in the rat: neurochemical correlates. Behav Brain Res. 1991;43:35–50. doi: 10.1016/s0166-4328(05)80050-6. [DOI] [PubMed] [Google Scholar]
- 39.McLean S, Grayson B, Harris M, Protheroe C, Woolley M, Neill J. Isolation rearing impairs novel object recognition and attentional set shifting performance in female rats. J Psychopharmacol. 2010;24:57–63. doi: 10.1177/0269881108093842. [DOI] [PubMed] [Google Scholar]
- 40.Jones CA, Brown AM, Auer DP, Fone KC. The mGluR2/3 agonist LY379268 reverses post-weaning social isolation-induced recognition memory deficits in the rat. Psychopharmacology (Berl) 2011;214:269–283. doi: 10.1007/s00213-010-1931-7. [DOI] [PubMed] [Google Scholar]
- 41.Bianchi M, Fone KF, Azmi N, Heidbreder CA, Hagan JJ, Marsden CA. Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. Eur J Neurosci. 2006;24:2894–2902. doi: 10.1111/j.1460-9568.2006.05170.x. [DOI] [PubMed] [Google Scholar]
- 42.Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- 43.Phillips GD, Harmer CJ, Hitchcott PK. Isolation rearing-induced facilitation of Pavlovian learning: abolition by postsession intra-amygdala nafadotride. Physiol Behav. 2002;76:677–684. doi: 10.1016/s0031-9384(02)00802-8. [DOI] [PubMed] [Google Scholar]
- 44.Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Wohr M, Schwarting RK. Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behav Neurosci. 2008;122:310–330. doi: 10.1037/0735-7044.122.2.310. [DOI] [PubMed] [Google Scholar]
- 46.Bredy TW, Barad M. Social modulation of associative fear learning by pheromone communication. Learn Mem. 2009;16:12–18. doi: 10.1101/lm.1226009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim EJ, Kim ES, Covey E, Kim JJ. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One. 2010;5:e15077. doi: 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiyokawa Y, Shimozuru M, Kikusui T, Takeuchi Y, Mori Y. Alarm pheromone increases defensive and risk assessment behaviors in male rats. Physiol Behav. 2006;87:383–387. doi: 10.1016/j.physbeh.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Fanselow MS. Odors released by stressed rats produce opioid analgesia in unstressed rats. Behav Neurosci. 1985;99:589–592. doi: 10.1037//0735-7044.99.3.589. [DOI] [PubMed] [Google Scholar]
- 50.Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753–765. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 51.Fried I, Cameron KA, Yashar S, Fong R, Morrow JW. Inhibitory and excitatory responses of single neurons in the human medial temporal lobe during recognition of faces and objects. Cereb Cortex. 2002;12:575–584. doi: 10.1093/cercor/12.6.575. [DOI] [PubMed] [Google Scholar]
- 52.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 53.Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 55.Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 56.Kuraoka K, Nakamura K. Responses of single neurons in monkey amygdala to facial and vocal emotions. J Neurophysiol. 2007;97:1379–1387. doi: 10.1152/jn.00464.2006. [DOI] [PubMed] [Google Scholar]
- 57.Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-expression and gaze-selective responses in the monkey amygdala. Curr Biol. 2007;17:766–772. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 58.Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- 59.Katayama T, Jodo E, Suzuki Y, Hoshino KY, Takeuchi S, Kayama Y. Phencyclidine affects firing activity of basolateral amygdala neurons related to social behavior in rats. Neuroscience. 2009;159:335–343. doi: 10.1016/j.neuroscience.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Kollack-Walker S, Newman SW. Mating-induced expression of c-fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. J Neurobiol. 1997;32:481–501. doi: 10.1002/(sici)1097-4695(199705)32:5<481::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 61.Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 63.Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol. 2003;33:13–29. doi: 10.1023/a:1021171129766. [DOI] [PubMed] [Google Scholar]
- 64.Gos T, Becker K, Bock J, Malecki U, Bogerts B, Poeggel G, Braun K. Early neonatal and postweaning social emotional deprivation interferes with the maturation of serotonergic and tyrosine hydroxylase-immunoreactive afferent fiber systems in the rodent nucleus accumbens, hippocampus and amygdala. Neuroscience. 2006;140:811–821. doi: 10.1016/j.neuroscience.2006.02.078. [DOI] [PubMed] [Google Scholar]
- 65.Guarraci FA, Frohardt RJ, Falls WA, Kapp BS. The effects of intra-amygdaloid infusions of a D2 dopamine receptor antagonist on Pavlovian fear conditioning. Behav Neurosci. 2000;114:647–651. doi: 10.1037//0735-7044.114.3.647. [DOI] [PubMed] [Google Scholar]
- 66.Nader K, LeDoux JE. Inhibition of the mesoamygdala dopaminergic pathway impairs the retrieval of conditioned fear associations. Behav Neurosci. 1999;113:891–901. doi: 10.1037//0735-7044.113.5.891. [DOI] [PubMed] [Google Scholar]
- 67.Lamont EW, Kokkinidis L. Infusion of the dopamine D1 receptor antagonist SCH 23390 into the amygdala blocks fear expression in a potentiated startle paradigm. Brain Res. 1998;795:128–136. doi: 10.1016/s0006-8993(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 68.Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- 69.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


