Figure 2.
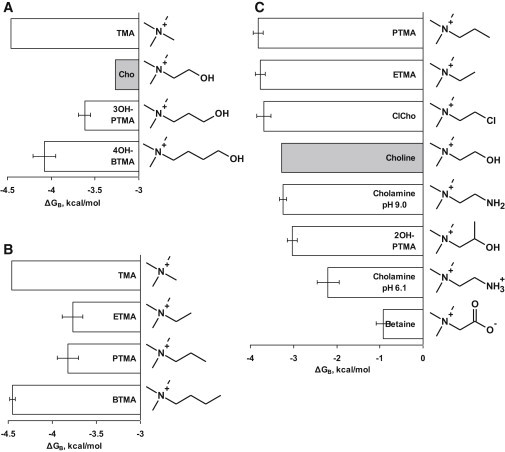
Binding energies and chemical structures of three sets of choline derivatives. (A) Extending the hydroxyethyl tail of choline to hydroxypropyl and hydroxybutyl (TMA shown for comparison). (B) Extending the alkyl tail of TMA from methyl to ethyl, propyl, or butyl. (C) Substituting the hydroxyl group of choline at C2 with different functional groups. Ligands with H-bonding ability are weaker agonists.
