Figure 4.
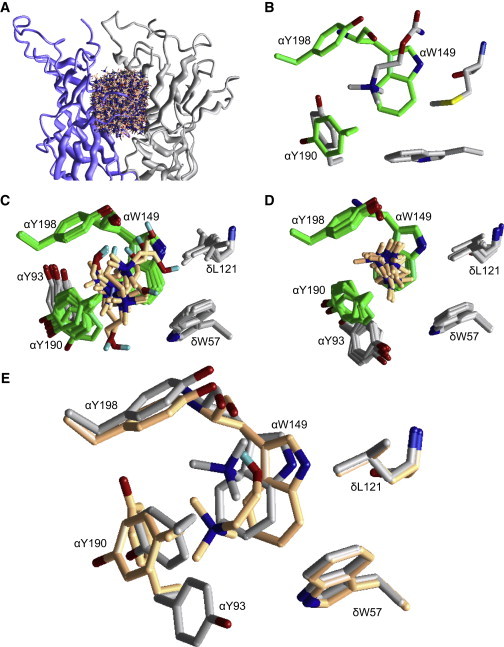
AChBP-based homology model of the AChR transmitter binding site with TMA and choline. (A) 1000 of the 10,000 randomly sampled orientations and positions of choline within the AChR transmitter binding site. The backbones of the α and δ subunits are presented as smooth ribbons. The ligand is shown in wireframe format, but its ammonium group is shown as a dark ball. (B) X-ray structure of Lymnaea AChBP bound with carbamylcholine (PDB accession number 1uv6). The aromatic triad is in green (AChR numbering). (C) The 10 most favorable binding modes of choline. Only residues within 4 Å from the ligand are shown as sticks. For clarity, the backbone atoms of the highlighted residues are not shown, except for αW149 and δL121. In six of the 10 modes, there is an H-bond between the choline hydroxyl and the backbone carbonyl of αW149. (D) The 10 most favorable binding modes of TMA. (E) Comparison of favorable binding modes of choline versus TMA. The relative position of the quaternary ammonium group within the aromatic triad (αW149, αY190, and αY198) is different for these two agonists. Although αY93 adopts alternative rotamers in TMA versus choline, this aromatic group likely contributes little to ΔGB.
