Figure 3.
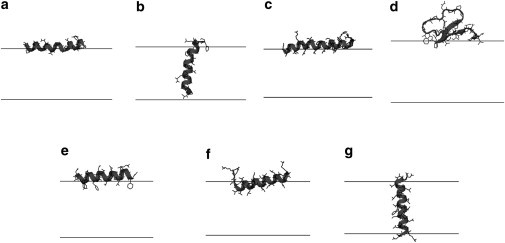
The average configuration of (a) interfacial alamethicin, (b) transmembrane alamethicin, (c) melittin, (d) KB1, (e) 18A, (f) interfacial KKpL15, and (g) transmembrane KKpL15 at nP/nL = 0.01 and χ = 0. The average helix contents calculated based on the structures from our simulations are: 85% for alamethicin, 80% for melittin, 84% for 18A, and 75% for KKpL15, which are a little higher than the corresponding experimental values of 71–74% (84), 76% (84), 70–76% (76), and 65–75% (77). The structures do not change significantly with nP/nL or χ. The lines denote the hydrophobic boundary of the bilayers.
