Abstract
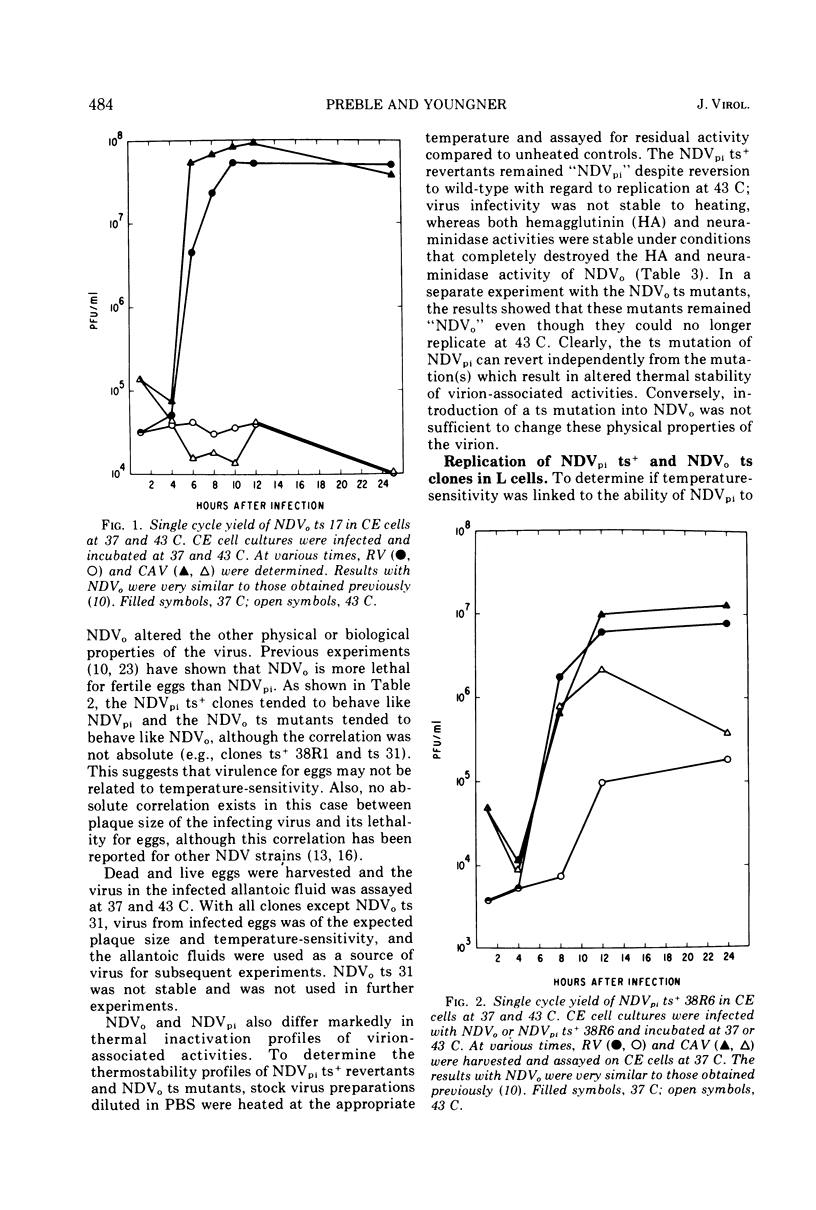
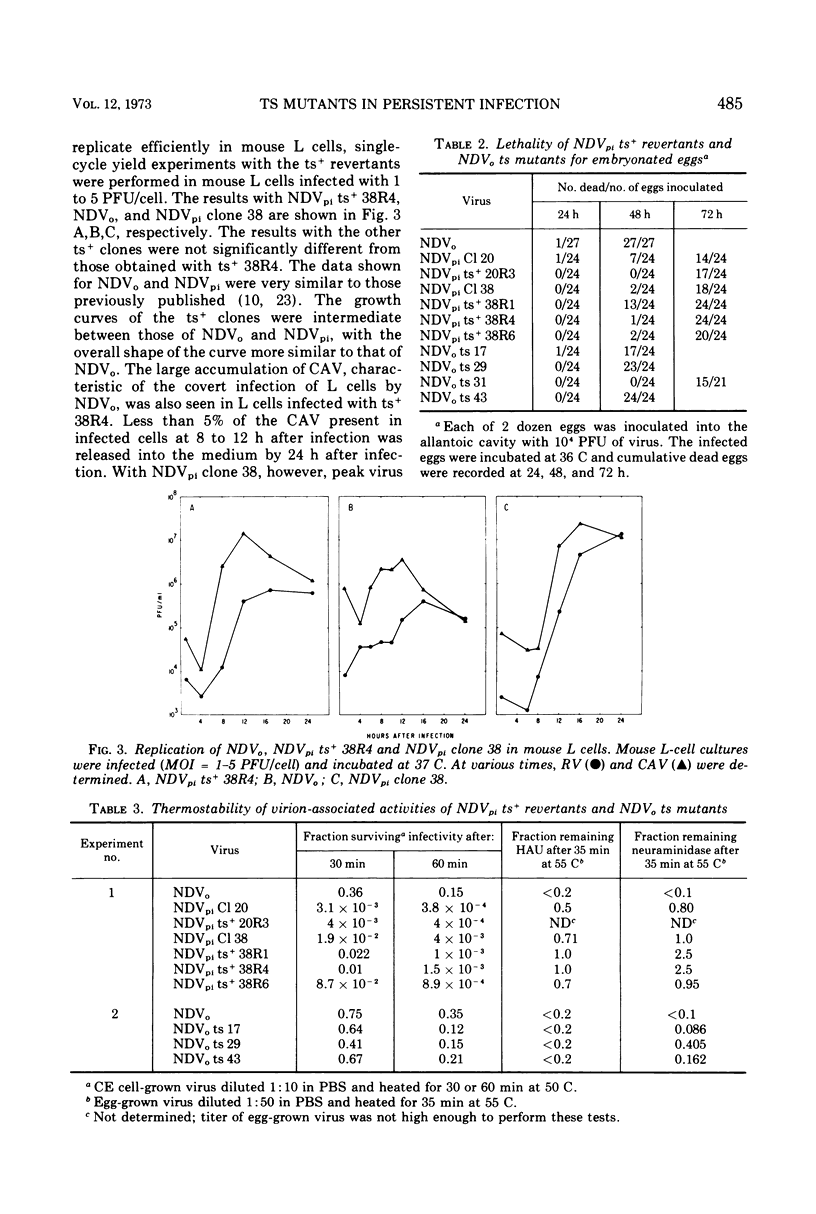
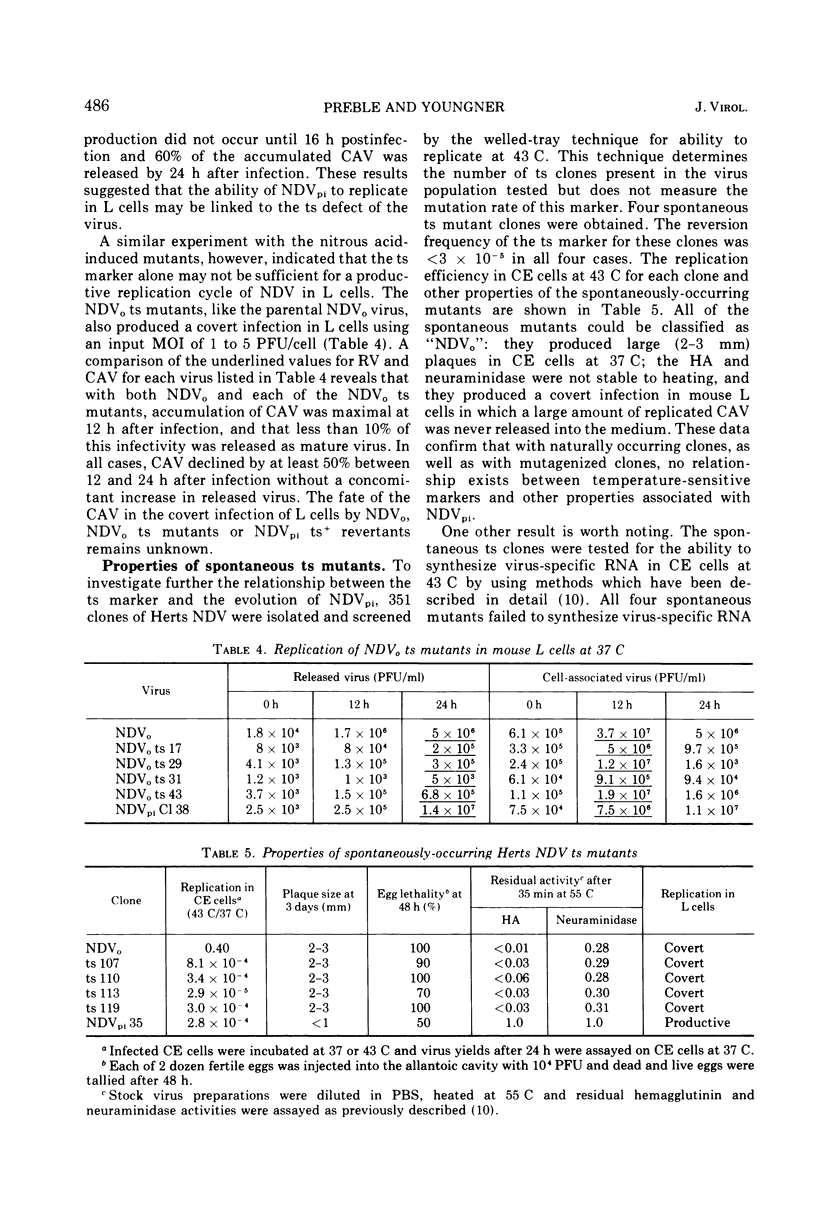
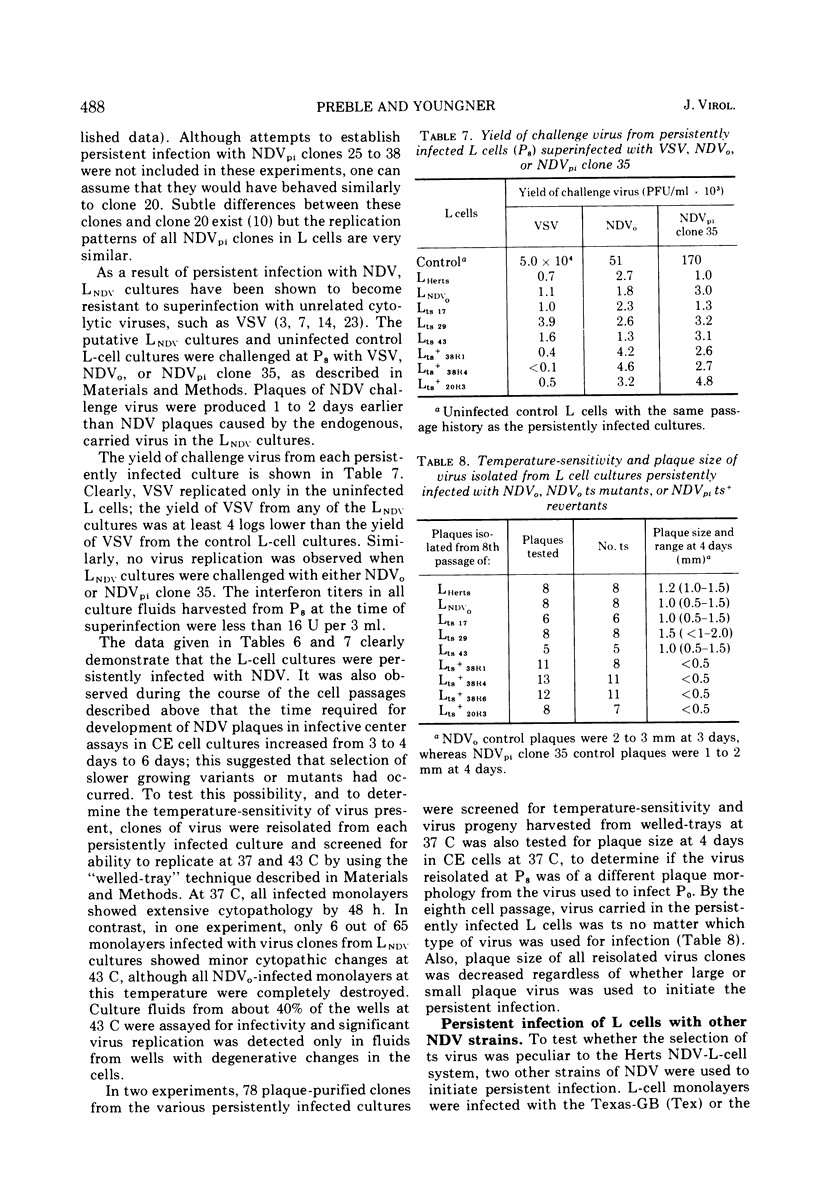
Virus mutants (NDVpi) recovered from L cells persistently infected with Newcastle disease virus (NDV, Herts strain) are temperature-sensitive (ts) at 43 C, although the wild-type virus (NDVo) which initiated the persistent infection replicates normally at that temperature. To study the relationship between the ts marker of NDVpi and the other properties which distinguish this virus from NDVo, NDVpi ts+ revertants were selected at the nonpermissive temperature and NDVo ts mutants were generated by treating NDVo with nitrous acid. Spontaneously-occurring ts mutants in the Herts NDV population were also isolated. The different virus populations were characterized with regard to plaque size, virulence for eggs, and thermal stability of infectivity, hemagglutinin, and neuraminidase. The NDVpi ts+ revertants, although no longer temperature-sensitive, retained NDVpi properties, whereas both spontaneously-occurring and mutagen-induced ts mutants remained wild-type in their other properties. These findings showed that the properties which characterized NDVpi were independent of the ts marker. However, the ts marker and the other markers of NDVpi were coselected during the persistent infection, and the combination of those markers appeared to be important in the outcome of NDV infection of L cells. NDVpi replicated productively in L cells, whereas NDVo, the NDVpi ts+ revertants, and the spontaneously-occurring ts mutants all yielded covert infections in L cells. The role of the selection of ts mutants in persistent infection was confirmed as follows: L cells were persistently infected with NDVpi ts+ revertants and NDVo ts mutants. Virus recovered from the persistently infected cultures after eight cell passages was always temperature-sensitive and of smaller plaque size than the parental virus in chicken embryo cell cultures. Similar results were obtained with virus recovered from L-cell cultures persistently infected with two other velogenic strains of NDV, the Texas-GB and Kansas-Man strains. These results strongly suggest that selection of ts mutants during the persistent infection was not random and played a role in establishment or maintenance of the persistent infection, or both.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- DEINHARDT F., BERGS V. V., HENLE G., HENLE W. Studies on persistent infections of tissue cultures. III. Some quantitative aspects of host cell-virus interactions. J Exp Med. 1958 Oct 1;108(4):573–589. doi: 10.1084/jem.108.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Simon E. H. Recombination in Newcastle disease virus (NDV): the problem of complementing heterozygotes. Virology. 1969 Jul;38(3):490–493. doi: 10.1016/0042-6822(69)90165-2. [DOI] [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- GRANOFF A. Induction of Newcastle disease virus mutants with nitrous acid. Virology. 1961 Apr;13:402–408. doi: 10.1016/0042-6822(61)90270-7. [DOI] [PubMed] [Google Scholar]
- Gavrilov V. I., Asher D. M., Vyalushkina S. D., Ratushkina L. S., Zmieva R. G., Tumyan B. G. Persistent infection of continuous line of pig kidney cells with a variant of the WSN strain of influenza A 0 virus. Proc Soc Exp Biol Med. 1972 May;140(1):109–117. doi: 10.3181/00379727-140-36405. [DOI] [PubMed] [Google Scholar]
- HENLE W., HENLE G., DEINHARDT F., BERGS V. V. Studies on persistent infections of tissue cultures. IV. Evidence for the production of an interferon in MCN cells by myxoviruses. J Exp Med. 1959 Oct 1;110:525–541. doi: 10.1084/jem.110.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirvaitis J., Simon E. H. A radiobiological study of the development of Newcastle disease virus. Virology. 1965 Aug;26(4):545–553. doi: 10.1016/0042-6822(65)90316-8. [DOI] [PubMed] [Google Scholar]
- Nagata I., Kimura Y., Ito Y., Tanaka T. Temperature-sensitive phenomenon of viral maturation observed in BHK cells persistently infected with HVJ. Virology. 1972 Aug;49(2):453–461. doi: 10.1016/0042-6822(72)90497-7. [DOI] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive defect of mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1973 Sep;12(3):472–480. doi: 10.1128/jvi.12.3.472-480.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1972 Feb;9(2):200–206. doi: 10.1128/jvi.9.2.200-206.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B. Preliminary physiological characterization of temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Jul;8(1):56–61. doi: 10.1128/jvi.8.1.56-61.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ J. E., HENLE W. STUDIES ON PERSISTENT INFECTIONS OF TISSUE CULTURES. V. THE INITIAL STAGES OF INFECTION OF L(MCN) CELLS BY NEWCASTLE DISEASE VIRUS. J Exp Med. 1964 Jan 1;119:895–921. doi: 10.1084/jem.119.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve P., Poste G. Studies on the cytopathogenicity of Newcastle disease virus: relation between virulence, polykaryocytosis and plaque size. J Gen Virol. 1971 Apr;11(1):17–24. doi: 10.1099/0022-1317-11-1-17. [DOI] [PubMed] [Google Scholar]
- Schloer G. M., Hanson R. P. Relationship of plaque size and virulence for chickens of 14 representative Newcastle disease virus strains. J Virol. 1968 Jan;2(1):40–47. doi: 10.1128/jvi.2.1.40-47.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwöbel W., Ahl R. Peristence of sindbis virus in BHK-21 cell cultures. Arch Gesamte Virusforsch. 1972;38(1):1–10. doi: 10.1007/BF01241350. [DOI] [PubMed] [Google Scholar]
- Simizu B., Takayama N. Isolation of two plaque mutants of Western equine encephalitis virus differing in virulence for mice. J Virol. 1969 Nov;4(5):799–800. doi: 10.1128/jvi.4.5.799-800.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simizu B., Takayama N. Relationship between neurovirulence and temperature sensitivity of an attenuated western equine encephalitis virus. Arch Gesamte Virusforsch. 1971;35(2):242–250. doi: 10.1007/BF01249716. [DOI] [PubMed] [Google Scholar]
- Straver P. J., van Bekkum J. G. Plaque production by carrier strains of foot-and-mouth disease virus in BHK-monolayers incubated at different temperatures. Arch Gesamte Virusforsch. 1972;37(1):12–18. doi: 10.1007/BF01241146. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., HABEL K. Virus-cell relationship in a carrier culture of HeLa cells and Coxsackie A9 virus. Virology. 1959 Jan;7(1):28–44. doi: 10.1016/0042-6822(59)90175-8. [DOI] [PubMed] [Google Scholar]
- Tan K. B., Sambrook J. F., Bellett A. J. Semliki forest virus temperature-sensitive mutants: isolation and characterization. Virology. 1969 Jul;38(3):427–439. doi: 10.1016/0042-6822(69)90155-x. [DOI] [PubMed] [Google Scholar]
- Thacore H. R., Youngner J. S. Cells persistently infected with Newcastle disease virus. 3. Thermal stability of hemagglutinin and neuraminidase of a mutant isolated from persistently infected L cells. J Virol. 1971 Jan;7(1):53–58. doi: 10.1128/jvi.7.1.53-58.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with Newcastle disease virus. II. Ribonucleic acid and protein synthesis in cells infected with mutants isolated from persistently infected L cells. J Virol. 1970 Jul;6(1):42–48. doi: 10.1128/jvi.6.1.42-48.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with newcastle disease virus: I. Properties of mutants isolated from persistently infected L cells. J Virol. 1969 Sep;4(3):244–251. doi: 10.1128/jvi.4.3.244-251.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. L. THE VIRAL CARRIER STATE IN ANIMAL CELL CULTURES. Prog Med Virol. 1964;6:111–148. [PubMed] [Google Scholar]
- Youngner J. S., Scott A. W., Hallum J. V., Stinebring W. R. Interferon production by inactivated Newcastle disease virus in cell cultures and in mice. J Bacteriol. 1966 Oct;92(4):862–868. doi: 10.1128/jb.92.4.862-868.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


