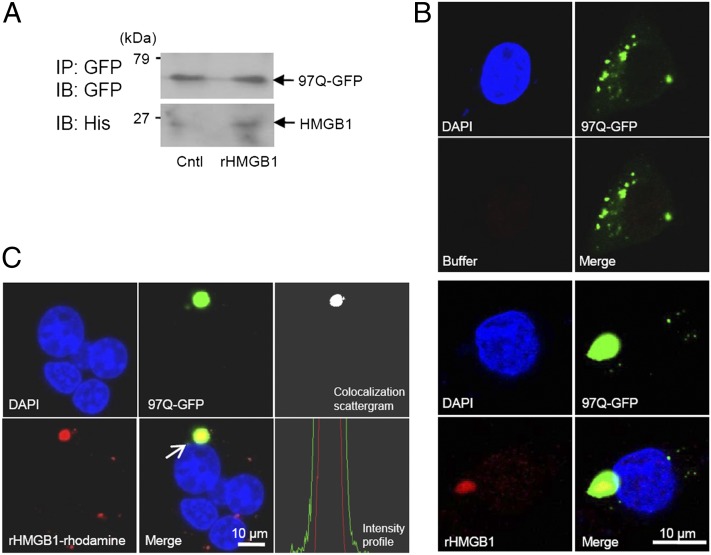
FIGURE 6.
Interaction of exogenously treated rHMGB1 with 97Q-GFP. (A) SHSY5Y cells were transiently transfected with 97Q-GFP plasmid and then treated with rHMGB protein at 4 μg/ml. SHSY5Y cell lysates were immunoprecipitated (IP) with anti-GFP Ab, and the binding of rHMGB1 protein to 97Q-GFP was observed with anti-His Ab (immunoblot [IB]). (B) SHSY5Y cells were transiently transfected with 97Q-GFP plasmid and then treated with rHMGB protein at 4 μg/ml. The cells were immunostained with anti-His Ab for intracellular staining of rHMGB1 protein, which was treated to the cell-culture medium (bottom panel). Buffer-treated control is shown in the upper panel. (C) SHSY5Y cells were transiently transfected with 97Q-GFP and then treated with rhodamine-conjugated rHMGB1 protein at a concentration of 4 μg/ml to detect direct interaction with 97Q-GFP protein. The colocalization of 97Q-GFP and rhodamine-rHMGB1 was observed using colocalization scatter plot and intensity profile analysis, which was measured across the aggregate (arrow). Cntl, Control.