Table 1.
Reactions of 2-(Phenylsulfonyl)-1,3-oxazole 1
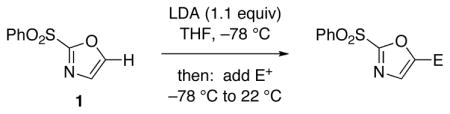
| |||
|---|---|---|---|
| entry | electrophile | producta,b | yieldc (%) |
| 1 |
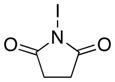
|
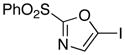 6
6
|
87 |
| 2 |
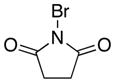
|
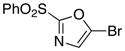 7
7
|
81 |
| 3 | n-Bu3SnCl |
|
86 |
| 4 | CH3I |
|
91 |
| 5 |
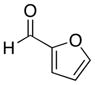
|
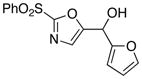 10
10
|
72 |
| 6 |
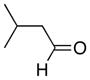
|
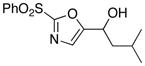 11
11
|
75 |
| 7 |
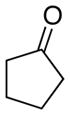
|
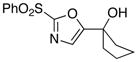 12
12
|
87 |
| 8 |
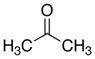
|
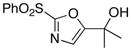 13
13
|
81 |
Reaction conditions: A THF solution of sulfone 1 (1.0 equiv; 2.0 mmol) was added into a solution of LDA (1.1 equiv) in THF at −78 °C. After stirring under N2 for 1 h, electrophile in THF solution was added with subsequent warming to 22 °C.
Products were purified by flash silica gel chromatography.
Yields are reported for isolated and purified product.
