Table 2.
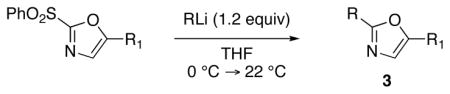
Preparation of 2,5-Disubstituted-1,3-oxazoles 3 via Displacement of the 2-Phenylsulfonyl Substituent
| ||||
|---|---|---|---|---|
| entry | lithium reagent | sulfone | oxazole producta,b | yieldc (%) |
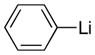
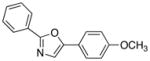
| 1 |
|
|
 19
19
|
90 |
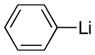
| 2 |
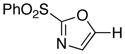
|
 10
10
|
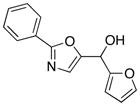 20
20
|
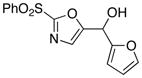
85 |
| 3 |
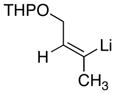
|
 1
1
|
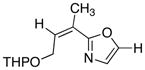 21
21
|
78 |
| 4 |
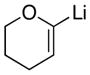
|
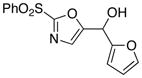 10
10
|
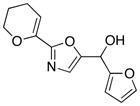 22
22
|
71 |
| 5 |
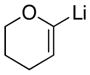
|
|
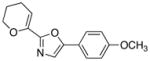 23
23
|
83 |
| 6 |
|
|
|
69 |
| 7 |
|
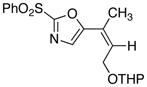 17
17
|
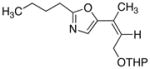 25
25
|
76 |
| 8 |
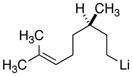
|
|
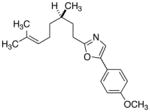 26
26
|
79 |
| 9 |
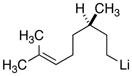
|
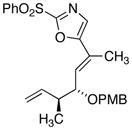 18d
18d
|
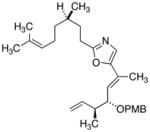 27
27
|
81 |
| 10 |
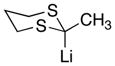
|
|
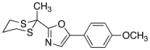 28
28
|
82 |
Reaction conditions: Lithium reagents (1.2 equiv or 2.2 equiv in entries 2 and 4) were added under N2 to a THF solution of sulfones (0.053 mmol) at 0 °C. Reactions were generally complete within 5 minutes and warmed to 22 °C prior to addition of aq. NH4Cl.
Products were purified by flash silica gel chromatography.
All yields are reported for isolated and purified products.
Compound 18 was prepared via the Stille cross-coupling reaction as described for 17.
