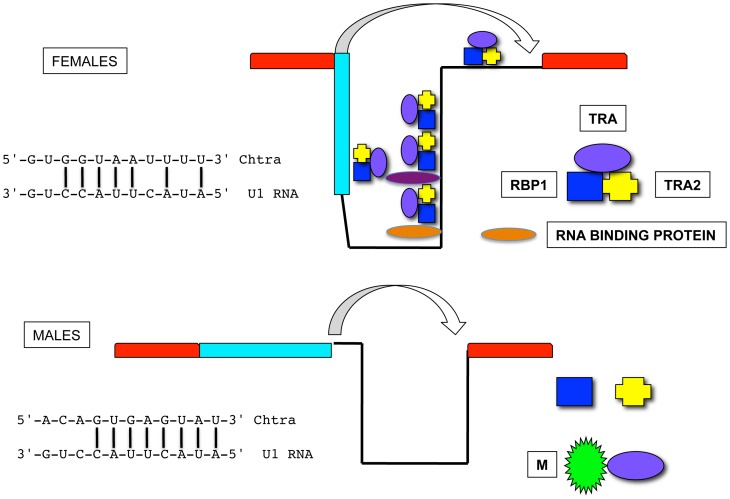
Figure 9. Proposed model for regulation of tra splicing in calliphorids.
The male splice donor site is preferred in males, possibly because this forms a stronger predicted hybrid with U1 snRNA (Drosophila sequence shown) than the female donor site. M is suggested to bind to the conserved amino terminal domain of TRA and prevent assembly of the TRA/TRA2/RBP1 complex. See discussion for alternative mechanisms by which M could regulate tra splicing. In females the TRA/TRA2/RBP1 complex binds to the single site in the male exon and the cluster of sites in the intron. We propose that intermolecular interactions between bound complexes block use of the male splice donor site. This interaction may be facilitated by additional RNA binding proteins that bind to conserved motifs (Figures 4,5). A TRA/TRA2/RBP1 complex is suggested to bind to the conserved motif (Figure 4B) near the 3′ splice acceptor site and enhance splicing with the female splice donor site.