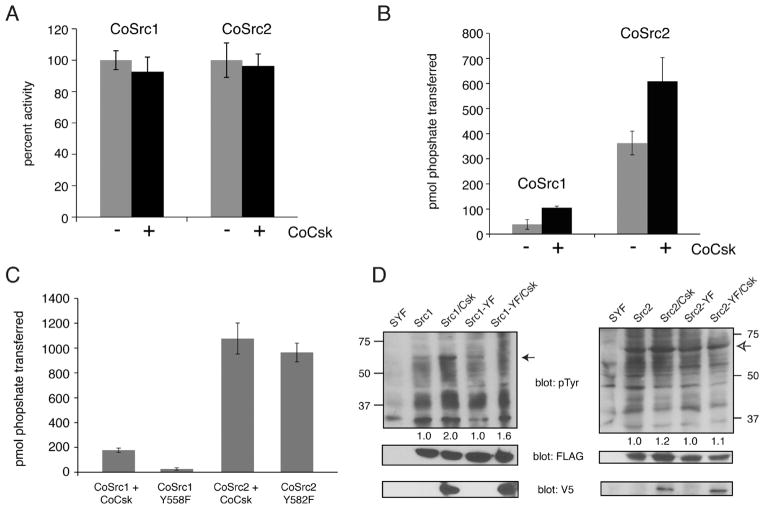
Figure 8.
CoCsk does not inhibit CoSrc Activity. (A) In vitro CoSrc kinase assays were performed in the presence or absence of purified CoCsk. Reaction mixtures contained 500 nM CoSrc1 or CoSrc2, CoCsk (600 nM), 500 μM [γ-32P]ATP, and RCM-lysozyme (0.4 mg/mL) as a Src substrate. After 20 min at 30 °C, reaction mixtures were analyzed by scintillation counting, as described in Materials and Methods. For each enzyme, activity without CoCsk was normalized to 100%. (B) CoSrc1 or CoSrc2 was expressed in SYF cells in the presence and absence of CoCsk. CoSrc proteins were immunoprecipitated with immobilized FLAG antibody and incubated with Src substrate peptide and [γ-32P]ATP. After 20 min at 30 °C, activity was measured using the phosphocellulose paper assay. (C) Similar experiments were conducted to compare wild-type CoSrc1 and CoSrc2 (isolated from SYF cells co-expressing CoCsk) with mutant forms lacking C-terminal tyrosines. In each case, equivalent immunoprecipitation of CoSrc kinases was verified by anti-FLAG Western blotting (data not shown). (D) Western blot analyses of CoSrc1 (left) or CoSrc2 (right) activity (wild-type or C-terminal tail YF mutants) in the presence or absence of CoCsk. Whole cell lysates of SYF cells were analyzed by anti-pTyr blotting. The filled arrow denotes the position of CoSrc1; the empty arrow denotes the position of CoSrc2. Equal expression of CoSrc1 and CoSrc2 was confirmed by anti-FLAG blotting and equal expression of CoCsk by anti-V5 blotting. Quantitation was performed as described in the legend of Figure 6.