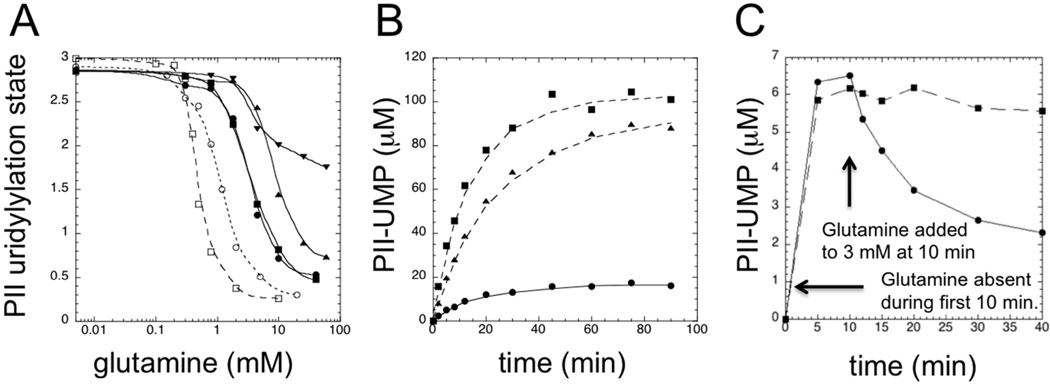
Figure 1. A his-tagged version of the UTase/UR was defective in steady-state glutamine signaling in reconstituted PII-UTase/UR covalent modification cycles.
A. Steady state glutamine responses of reconstituted cycles. Experiments were conducted as in Materials and Methods, and the steady-state levels of PII modification at various glutamine concentrations are shown, stated in terms of modified subunits/tetramer. Symbols: □, PII at 36 µM and wt UTase/UR at 1.2 µM; ○, PII at 0.5 µM and wt UTase/UR at 0.02 µM; ▼, PII at 36 µM and his-tagged UTase/UR (UQ5516) at 1.2 µM; ▲, PII at 3 µM and his-tagged UTase/UR (UQ5516) at 0.1 µM; ■, PII at 0.5 µM and his-tagged UTase/UR (UQ5516) at 0.017 µM; ●, PII at 0.2 µM and his-tagged UTase/UR (UQ5516) at 0.0067 µM. B. Approach to the steady state in reconstituted systems containing 10 mM glutamine. All systems contained 36 µM PII and 1.2 µM enzyme. Symbols: ●, wt UTase/UR; ▲, his-tagged UTase/UR (UQ5516); ■, his-tagged UTase/UR (SA1). C. Response of reconstituted covalent modification cycles to the addition of glutamine. Systems contained 3 µM PII and 0.2 µM enzyme. For the first 10 min, systems were incubated in the absence of glutamine, after which glutamine was added to a final concentration of 3 mM. Symbols: ●, wt UTase/UR; ■, his-tagged UTase/UR (SA1).