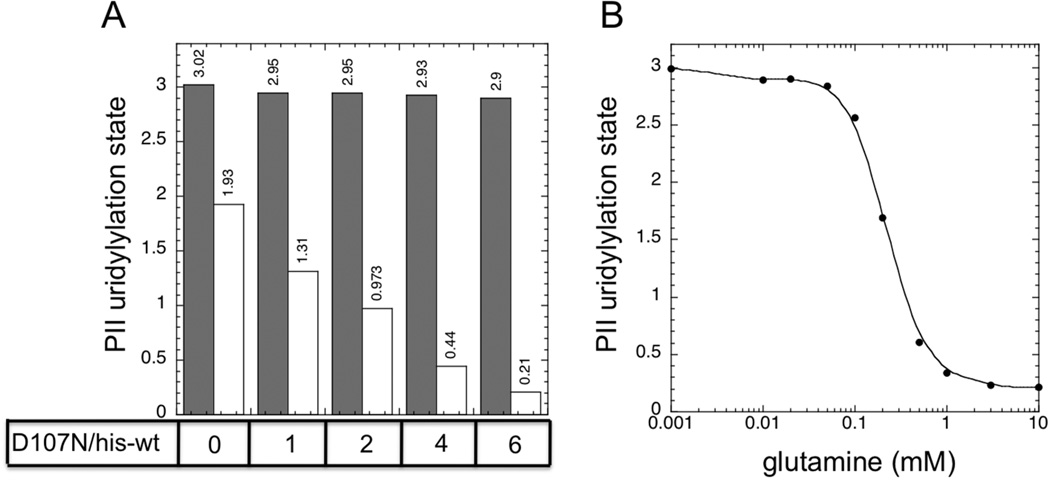
Figure 3. A combination of his-tagged UTase/UR and an altered form of the enzyme displaying only UR activity effectively regulated PII uridylylation state in response to glutamine.
A. Steady state PII uridylylation state in the absence of glutamine (filled bars) and in the presence of 10 mM glutamine (unfilled bars). PII was at 36 µM, his-tagged UTase/UR (UQ5516) was at 0.5 µM, and the altered D107N UTase/UR was present at 0 µM, 0.5 µM, 1 µM, 2 µM or 3 µM to provide the indicated ratios of enzymes. B. Glutamine signaling by a reconstituted UTase/UR-PII cycle containing a combination of his-tagged UTase/UR (UQ5516) (0.5 µM) and the altered D107N UTase/UR (3 µM). PII was present at 36 µM.