Abstract
Many species navigate in three dimensions and are required to maintain accurate orientation while moving in an Earth vertical plane. Here we explored how head direction (HD) cells in the rat anterodorsal thalamus responded when rats locomoted along a 360° spiral track that was positioned vertically within the room at the N, S, E, or W location. Animals were introduced into the vertical plane either through passive placement (experiment 1) or by allowing them to run up a 45° ramp from the floor to the vertically positioned platform (experiment 2). In both experiments HD cells maintained direction-specific firing in the vertical plane with firing properties that were indistinguishable from those recorded in the horizontal plane. Interestingly, however, the cells' preferred directions were linked to different aspects of the animal's environment and depended on how the animal transitioned into the vertical plane. When animals were passively placed onto the vertical surface, the cells switched from using the room (global cues) as a reference frame to using the vertically positioned platform (local cues) as a reference frame, independent of where the platform was located. In contrast, when animals self-locomoted into the vertical plane, the cells' preferred directions remained anchored to the three-dimensional room coordinates and their activity could be accounted for by a simple 90° rotation of the floor's horizontal coordinate system to the vertical plane. These findings highlight the important role that active movement signals play for maintaining and updating spatial orientation when moving in three dimensions.
Keywords: directional heading, head direction cells, navigation, spatial orientation, vertical plane
successful spatial navigation requires knowledge about one's current location and directional heading. Spatial orientation in rats is believed to be encoded by three major types of neurons: hippocampal place cells, entorhinal grid cells, and head direction (HD) cells, which have been identified in several areas within the limbic system (for reviews see Moser et al. 2008; Sharp et al. 2001; Taube 2007). HD cells are neurons that discharge as a function of the animal's head direction in the horizontal plane, independent of the animal's ongoing behavior (Taube et al. 1990a). The direction at which the cell fires maximally is referred to as the preferred firing direction.
Most experiments on HD cells have examined their responses while the animal locomotes within the horizontal plane. More recently, researchers have begun to monitor how these cells respond in three dimensions and in planes other than Earth horizontal. Stackman et al. (2000) monitored HD cells as rats climbed up and down a vertical ladder that was positioned at different locations relative to the cell's preferred direction. The authors reported that direction-specific firing continued in the vertical plane and that the cell's preferred direction was dependent on 1) its preferred orientation in the horizontal plane and 2) the orientation of the vertical surface relative to the surrounding environment. The results were consistent with the hypothesis that HD cells define the horizontal reference frame as the animal's plane of locomotion and treat the vertical plane as an extension of the floor's x-y coordinate system rotated by 90° (see Fig. 1). Another study monitored HD cell activity as the rat traveled a circular pattern that included two trips through the vertical plane (floor → wall → ceiling → wall → floor) and similarly found that HD cells responded on the walls as if they were an extension of the floor's coordinate system (Calton and Taube 2005). For both of these studies, when the rat was on the vertical surface the monitoring of HD cell activity was limited to a narrow range of directions that was aligned with the rat's longitudinal body axis, because the behavioral tasks did not require the animal to look around while it was traversing the walls up or down. Thus the first goal of the present study was to monitor HD cell activity in a task that required the animal to sample all 360° of directions when locomoting in the vertical plane, which would enable a more accurate comparison of HD cell properties across different planar surfaces.
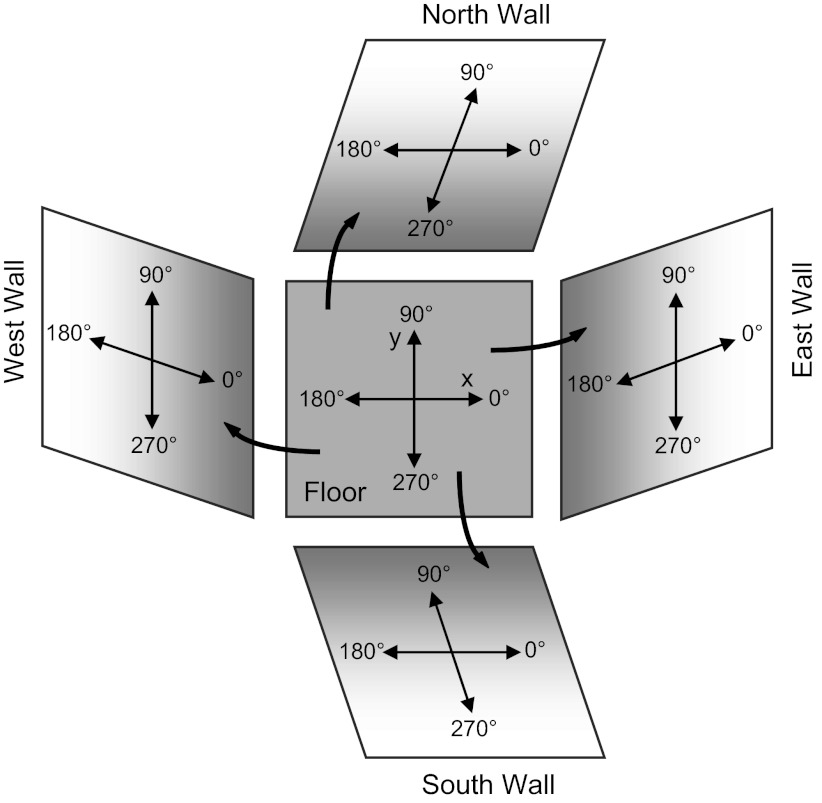
Fig. 1.
Coordinate system and reference frame. The x-y coordinate system is shown on the room floor and as an animal might rotate it by 90° as it locomotes onto the vertical surface along each of the 4 walls in the room. In this figure the orientation of the coordinate system denoted on the floor is rotated by 90° to obtain the coordinate system on each of the vertical walls.
When moving through the environment, a rat uses information derived from both external (landmark) and internal cues in order to maintain orientation. Internal (idiothetic) cues are spatial information derived from the animal's self-motion through the environment and include vestibular, motor efference, and proprioceptive signals. For this information to remain accurate it must be continually updated as the animal changes its position and orientation in space (Gallistel 1990). HD cells use both types of cues for maintaining a consistent directional reference (Taube et al. 1990b; Taube and Burton 1995). The ability of different neurons to encode multiple reference frames simultaneously was demonstrated by Gothard et al. (1996), who reported that simultaneously recorded hippocampal place cells could be divided into subpopulations based on the use of different reference frames. These reference frames were defined either by the recording room environment (global cues) or by the animals' immediate surroundings (local cues). The use of reference frames to influence HD cell activity can also be considered in terms of the external and internal cues available to the animal. Thus a second goal of this study was to understand the nature of the reference frames used by HD cells when an animal switches from locomoting on a horizontal surface to locomoting in a vertical plane. Specifically, do HD cells use global or local cues to define their reference frame when the animal locomotes from a horizontal plane to a vertical plane, and does the set of cues used depend upon whether the animal actively locomotes this transition as opposed to being passively placed into the vertical plane?
The present study monitored HD cell activity as the animal traversed a 360° spiral track in the vertical plane that could be positioned along any of the four walls at different cardinal directions within the recording room (i.e., north, south, east, west). We report here that HD cells contained directional tuning curves in the vertical plane that were similar to those seen in the horizontal plane. Furthermore, we show that HD cells sometimes switched reference frames when locomoting between the two surfaces. The reference frame used by the cells depended on whether the animals were passively placed into the vertical plane or actively self-locomoted their transition between the horizontal and vertical planes. When animals were positioned in the vertical plane, the cells usually used the vertical platform the animals moved on as the reference frame (local cues). In contrast, when animals were self-locomoting into the vertical plane, the cells continued to use the room as the reference frame (global cues) rather than the vertical platform.
METHODS
Two different experiments were conducted on separate groups of animals. In experiment 1, animals were passively moved by hand between the horizontal and vertical planes. In experiment 2, animals actively locomoted between the two planes.
Subjects and Apparatus
Subjects for experiment 1 were six female Long-Evans rats, weighing 250–300 g at the start of the experiment. There were five rats weighing similar amounts for experiment 2. One rat was used for both experiments 1 and 2. Rats were placed on a food-restricted diet of ∼15 g/day, and water was available ad libitum. The animals were housed individually in transparent plastic cages and were maintained on a 12:12-h light-dark cycle. Training and HD cell screening occurred during sessions in which the rats foraged for food pellets in a cylinder (71 cm high, 77-cm diameter). A white sheet of cardboard attached to the inside wall of the cylinder covering ∼100° of arc served as the only intentional visual cue that could be used for orientation. With the room as a reference frame, this cue card was centered along the west side of the cylinder for all experiments. The cylinder was placed on a sheet of gray photographic backdrop paper, which was replaced after each recording session to remove olfactory cues from the previous session.
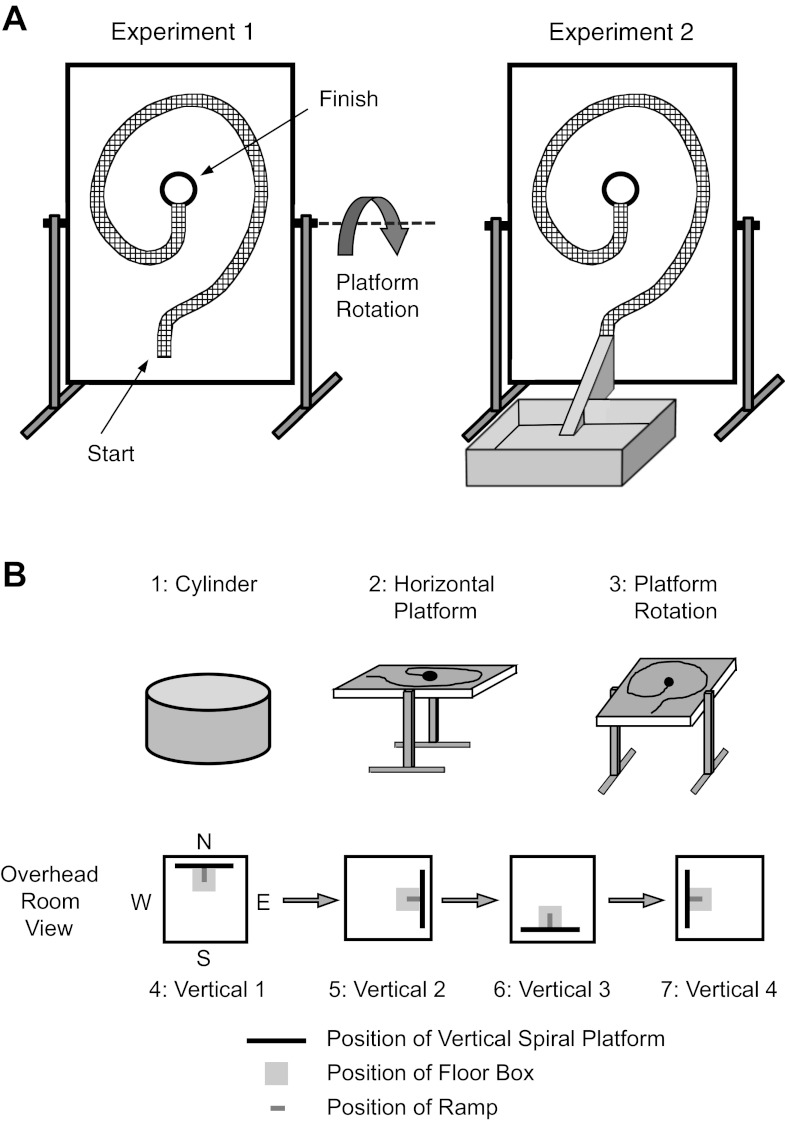
A second apparatus, a rectangular wooden platform, was used to monitor HD cell activity in the vertical plane. The wooden platform when vertical measured 1.20 m (width) × 1.53 m (height) and was adjustable such that it could be positioned at angles of 0°, 30°, 45°, and 90° relative to the floor (Fig. 2A), which was utilized for training the rats to climb on the wall (see pretraining below) and for testing. On the platform surface was a wire mesh (7.6 cm wide) that formed a spiral path that the rat could grasp as it locomoted along it. At the center of the spiral path was a circular escape hole (15-cm diameter), which the rat could pass through and find a horizontally positioned small platform (17.8 cm × 30.5 cm) that provided a resting location for the animal after each climbing session. A weigh boat holding food pellets was placed on the resting platform to provide motivational reward to reach the escape hole. When the platform was in the 0° (horizontal) position, its surface was ∼1 m above the floor (Fig. 2B2).
Fig. 2.
A, left: schematic of the spiral track platform in the vertical position. Right: the vertical platform along with the floor box and ramp used for experiment 2. B: protocol for the 7 consecutive recording sessions. See text for details. Black bar represents the position of the vertical platform within the recording room along with the floor box (light gray) and attached ramp (dark gray)(experiment 2). The positions of the vertical platform for experiment 1 were similar to those for experiment 2 but did not contain the floor box. Note: the start location for the first vertical wall session (session 4) was randomized between North, South, East, and West, and the sequence for the following Vertical sessions (sessions 5–7) was also randomized in terms of proceeding in a clockwise or counterclockwise direction.
The cylinder was surrounded by a black curtain 2.5 m in diameter that hung from the ceiling to the floor of the screening room. During the vertical wall and horizontal platform recording sessions, the curtain was pulled back to expose additional visual cues on the room walls, as well as the door to the room. Furthermore, the pulled-back curtains provided an additional stable room cue. Collectively, the room's doorway, various wall cues, and the pulled-back curtains provided a global set of landmark cues that remained present across all vertical wall sessions. Eight overhead DC lamps spaced equally in a circle provided room illumination. A color video camera (Sony SC-711) was attached to the ceiling directly above the cylinder. A second color video camera was attached to a tripod and positioned across from the vertical wall and was moved along with the vertical platform when the platform was positioned along different room walls.
Training Procedures
Rats were first trained to forage for food pellets in the cylinder. They were removed from their cages and placed in the cylinder for 10 min. During training the rats were encouraged to forage for small food pellets (20 mg; PJ Noyes, Lancaster, NH) dropped randomly into the cylinder from an automatic food pellet dispenser positioned above the apparatus. The procedure was repeated daily for 5–7 days. Upon completion of training, rats engaged in nearly continuous pellet search behavior. Rats for both experiments 1 and 2 underwent this training.
Once forage training in the cylinder was completed, rats for experiment 1 were trained to run along the spiral track on the platform apparatus. The wire mesh path was screwed onto the board with 11-cm-tall risers that allowed the path to be elevated from the board. The platform was first positioned at a 0° angle (horizontal), with 22 small food pellets placed along the spiral track and a large pellet (750 mg) placed at the end of the track. Once the rat was able to successfully locomote from the beginning to the end of the track within 30 s, the platform was repositioned at a 30° angle and only 11 small pellets were placed on the track to guide locomotion. When the rat was able to successfully locomote the path, the platform was elevated further to the 45° position and all food pellets were removed from the spiral path, except for the one large pellet placed at the end of the path. The training procedure was repeated until successful locomotion was accomplished. After successful performance with the platform positioned at 45°, the apparatus was positioned vertically (90°; Fig. 2A, left) and the risers on the platform were removed, such that the wire mesh track was flush against the platform. Training continued until the rat was able to locomote from the starting point of the spiral path at the bottom of the wooden platform to the end point at the center of the platform. At the center of the platform was the escape hole that the animal could climb through and find the large food pellet placed on the small horizontally positioned platform. Training for each step generally took 3–5 days. Once the rats were successfully performing the task, they then underwent surgery for implantation of recording electrodes.
For experiment 2, a wooden floor box and ramp were added to the apparatus. The training procedures were similar to those described above for experiment 1, except for the 90° vertical platform sessions where a 61-cm-square floor box was placed near the vertical platform, and a 45° ramp (7.6 cm wide, 41.5 cm long) was positioned between the floor box and the platform (Fig. 2A, right). Thus the rat could walk from the floor box to the starting point of the spiral path on the vertical platform via the ramp. When the rats were able to locomote from the floor box to the end point of the spiral path on the vertical wall, the training sessions were complete and the animals underwent surgery for implantation of recording electrodes.
Electrode Construction and Surgical Procedures
Electrode construction and implantation techniques used in this experiment were similar to those described previously (Taube 1995). Each recording electrode array consisted of a bundle of ten 25-μm-diameter nichrome wires (California Fine Wire, Grover City, CA) insulated except at the tips. The wire bundle was passed through a 26-gauge stainless steel cannula and each wire attached to a modified 11-pin Augat connector. The array of wires could be advanced in the dorsal-ventral plane with three screws attached to the electrode's acrylic base (Kubie 1984).
After training, each rat was anesthetized with Nembutal (1.0 ml/kg) and stereotaxically implanted with an electrode array positioned just above the anterodorsal thalamus. Electrode coordinates, with respect to bregma, were anterior/posterior −1.5 mm, medial/lateral +1.3 mm right, and ventral 3.7 mm from the cortical surface (Paxinos and Watson 1998). Four stainless steel screws were placed in the skull plates, and dental cement securely anchored the electrode assembly in place. All procedures were conducted in accordance with an institutionally approved animal care protocol and in compliance with guidelines described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Each rat was allowed a 1-wk postoperative recovery period before we began screening for HD cells.
Isolation and Recording of Head Direction Cell Activity
Each rat's microelectrodes were monitored for cellular activity while the rat foraged for food pellets in the cylinder. The rats were transported from the animal colony room into the screening room, and no attempt was made to disorient the rats before each session. Each of the 10 microelectrodes was monitored on an oscilloscope, and the rat's directional heading was observed on a video screen via the overhead video camera. The electrode wires were advanced gradually over several weeks while screening for HD cells, in which their electrical waveform was of sufficient amplitude for isolation from background noise. We monitored neural activity by passing the signals through a field-effect transistor (FET) that was in a source-follower configuration and then through an overhead commutator (Biela Idea Development, Anaheim, CA) to an amplifier (Grass Instruments P5 Series, West Warwick, RI). Signals were referenced to electrical activity recorded on a nearby wire and then differentially amplified (20,000), band-pass filtered (300–10,000 Hz, 3 dB/octave; Peavey Electronics PME8, Meridian, MS), and sent through a series of window discriminators (BAK Electronics model DDIS-1, Germantown, MD). The final signal was then displayed on an oscilloscope (Tektronix model 2214, Beaverton, OR). If no HD cell activity was detected, the entire electrode array was advanced 25–50 μm ventrally and electrical activity was screened again 24 h later. Screening for HD cells generally occurred over the course of 2–3 mo.
When the waveform of a HD cell could be isolated successfully, two light-emitting diodes (LEDs) were activated via the recording cable to monitor the animal's HD via the overhead video camera. The LED arrangement used in the present study was a modification of one used previously (Taube et al. 1990a). The headstage included a red LED positioned ∼1 cm posterior to the headstage and a green LED positioned ∼6 cm further away over the rat's back. The x,y-coordinates of the LEDs were monitored at 60 Hz by an automated video-tracking system (Ebtronics, Brooklyn, NY). During recording sessions the LED coordinates and the neural activity were acquired by a data acquisition interface board (National Instruments DIO-96, Austin, TX) in a Macintosh computer. The cell's firing rate relative to the animal's HD was computed with programs written with LabVIEW software (National Instruments).
Recording Protocol
All recording sessions took place in the same testing room. Distinct visual cues within the room provided landmarks the animals could use as a reference for orientation. These cues included a painting on the North wall, a floor-to-ceiling black curtain on the east side of the room that spanned ∼2 m of arc, and light coming from the open door in the northwest corner of the room. The animal remained connected to the recording cable between sessions, and no attempt was made to disorient the animal between recording sessions.
If cell isolation remained satisfactory throughout recording, then each HD cell was recorded over seven sessions (Fig. 2B). The cell's activity was first recorded for 8 min while the rat foraged for food pellets inside the cylinder (referred to as Cylinder session). This session established the cell's baseline preferred firing direction. After this baseline session, the rat was placed nearby in its transparent home cage (which had been brought into the recording room and placed on the floor without its top). The cage was generally positioned within 1–2 m of the room's center, but its location was not fixed within the room across sessions. Because there was no top to the cage when it was in the room, the rat could view the walls and ceiling of the room from inside this “holding” cage. The platform was set to a 0° angle (parallel to the floor) and positioned within the room such that the bottom end of the platform (which contained the beginning of the spiral) was parallel to one of the room walls. The rat was placed in the middle of the platform, and recording resumed for 4 min while the rat explored the platform (referred to as Horizontal-Platform session). Rats were encouraged to sample all 360° during this session by placing food pellets at random places on the platform. Afterwards, the rat was returned to the nearby holding cage, which lay on the room floor. To determine whether the cell's activity followed a room reference frame or a platform reference frame, a second horizontal session was conducted in which the platform was rotated 90° clockwise (CW) or counterclockwise (CCW) (referred to as Horizontal-Rotation session). The rat was returned to the center of the platform without disorienting it, and the cell was recorded for 4 min. The rat was then returned to the holding cage. For all periods between these recording sessions, and those described below for the Vertical sessions, the rat was always stationed in the holding cage on the room floor. The location of the holding cage within the room was not fixed and varied across sessions.
The next set of recording sessions involved recording the HD cell activity when the platform apparatus was positioned vertically adjacent to each of the four room walls (referred to as Vertical 1, 2, 3, or 4). One of the walls within the room (N, S, E, W) was selected randomly as the start position for the first Vertical session; thus there was no consistent relationship between the orientation of the horizontal platform sessions (Horizontal-Platform or Horizontal-Rotation) and the location of the first vertical wall session. After the first Vertical session (Vertical 1), the vertical platform was positioned at each of the other three walls, proceeding sequentially in either a CW or CCW direction (selected randomly) around the room. For example, in Fig. 2B the order of Vertical sessions is depicted as N, E, S, W. Between each Vertical session, the rat was returned to the holding cage for 3–4 min. For experiment 1 the rat was gently grasped by the experimenter, carried from the holding cage, which was stationed 1–2 m away, and placed at the start of the spiral track at the bottom of the vertical platform. The passive displacement of the rat generally took 2–3 s and went directly from the cage to the vertical platform without any intention to disorient it. HD cell activity was recorded while the rat locomoted along the spiral track from the starting point to the end point when it reached the reward platform. By recording cell activity from each of the four cardinal locations, responses could be compared across sessions to determine which coordinate frame the cells were using (i.e., room coordinates vs. platform coordinates).
For experiment 2, the recording procedure for the Cylinder, Horizontal-Platform, and Horizontal-Rotation sessions were identical to those described above. The Vertical sessions were also similar to those described above, except that the animal first locomoted around the floor box for 2 min before being allowed to climb the 45° ramp to the vertical spiral track. During this 2-min period, a few food pellets were placed on the floor of the box to encourage the animal to move about the box and forage for food. Two cameras were again used for each session. The HD cell was recorded while the rat locomoted inside the floor box and then as it moved up the ramp. As soon as the rat climbed onto the vertical spiral track, the video line was manually switched from the overhead camera to the tripod-mounted camera in order to track the LED positions as the rat locomoted around the vertical spiral track.
Data Analysis
For each recording session, a firing rate vs. HD tuning curve was plotted to determine the cell's directional tuning. HDs were sorted into one of forty 9° bins. The x-y coordinate system used by the tripod-mounted video camera had to be transformed for each of the vertical wall sessions in order to match the coordinate system used by the ceiling-mounted camera. We chose to define the coordinate system for the vertical wall positions by using the recording room as a reference frame (as viewed by the overhead video camera). Thus the amount that the tripod-mounted camera coordinate system had to be transformed to correspond to the room coordinate frame was dependent on the wall on which the vertical platform was positioned. The transformations for the North, South, East, and West walls were 180°, 0°, 270°, and 90°, respectively.
On the basis of the cell's firing rate vs. HD tuning curve, we monitored the cell's 1) preferred firing direction (the HD at which the cell fired maximally), 2) peak firing rate (the bin with the maximal firing rate), 3) background firing rate (the mean firing rate from all bins that were >18° away from the cell's directional firing range), and 4) directional firing range (the range of head directions in which the firing rate was elevated above background level; based on a triangular model, Taube et al. 1990a). To determine whether cell firing was significantly modulated by the animal's directional heading, we performed a Rayleigh test based on the cell's firing rate vs. HD tuning curve (Batschelet 1981). This statistical test first involves computing the mean vector length, r, based on the cell's firing over 360°, and is referred to as the Rayleigh r value when we describe the cell properties. Mean vector length is calculated by redrawing the tuning curve in polar coordinates and representing each of the firing rates as a vector. The mean vector is then determined with trigonometric functions. Mean vector lengths range between 0 and 1, with higher values indicating that spike occurrence is clustered around a particular direction. The critical significance level of r is then determined by the number of observations (which was defined as the sum of all firing rates from the 40 directional bins), and if the r value meets this significance level the distribution is considered to be nonrandom. Cells that exhibited significantly nonrandom distributions were considered directionally tuned. HD cells typically have Rayleigh r values > 0.5.
The amount that the preferred direction shifted between sessions was determined by calculating the correlation between the two firing rate vs. HD tuning curves and then rotating one tuning curve relative to the second tuning curve in 9° steps until the maximal correlation (rmax) was obtained. The amount that one tuning curve was shifted relative to the second tuning curve to obtain rmax was defined as the shift value between those two sessions. Mean shifts ± the mean angular deviation are expressed with circular statistics (Batschelet 1981), where the mean shift is expressed as an angle, Ø, with an associated mean vector length (rshift) that varies between 0 and 1. To determine the amount of variation (dispersion), we converted the r value to an angular value, using the formula [2(1 − rshift)]1/2. This angular value is comparable to the standard deviation used in linear statistics. We used a circular V-test to determine whether the observed values clustered around a particular angle (usually 0°), using the test statistic u (Batschelet 1981, p. 58–60). A significant value for u indicates that the observed values clustered around a particular angle. To compare shifts of the preferred firing direction between two different conditions, we used the Watson-Williams test, which computes an F value for the sample groups in question (Batschelet 1981, p. 95–101). This F value is then compared with critical F values in standard statistical tables. One assumption for this test is that the parameter of concentration (κ) has to be similar for both samples. This parameter is a measure of how much dispersion is present in the data and varies between 0 and 1, with decreasing values representing greater dispersion. Thus, prior to conducting the Watson-Williams test, we performed a parametric test for the concentration parameters to ensure their similarity (Batschelet 1981, p. 122–124). This test can also be used to determine whether the variability is similar or different between two groups. All significant values were defined at the 0.05 level.
For many recording sessions, more than one HD cell was recorded simultaneously. Because the preferred firing directions of HD cells usually remain in register with one another across sessions (Taube et al. 1990b; Taube 1995), we used the average shift across cells for a particular session as the unit measure rather than individual cells, in order to avoid unduly weighting sessions in which multiple cells were recorded. We also note that in all instances where multiple cells were recorded simultaneously in the present study (experiment 1: n = 1 pair; experiment 2: n = 5 pairs, 3 triplets, 1 quadruplet) shifts of the preferred direction in one cell were similar (within ±18°) to the shifts in the preferred directions of the other simultaneously recorded cells.
We also examined whether there were any changes in cell firing properties between sessions. Specifically, we compared sessions when the animal locomoted in the horizontal plane (Horizontal-Platform and Box sessions) with sessions on the vertical spiral track (Vertical 1–Vertical 4). For these comparisons, we evaluated changes for three parameters: 1) peak firing rate, 2) background firing rate, and 3) Rayleigh r value. To compare changes in these parameters across sessions we used a standard index score (ParameterSI), which was defined as
where Parameterfirst is the value for the cell's parameter in the first session and Parametersecond is the parameter's value in the second session. Standard index scores can range from −1 to +1, with values near 0 indicating little change in the parameter across sessions.
Histology
Upon completion of the experiments, all 10 animals were anesthetized deeply with Nembutal (1.0 ml/kg) and a small anodal current (20 μA, 10 s) was passed through one of the electrodes to later conduct a Prussian blue reaction on the tissue. The animals were then perfused transcardially with saline (2 min) followed by 10% formalin in saline (15 min). The brains were placed in 10% formalin for at least 48 h. Two days prior to brain sectioning, potassium ferrocyanide was added to the 10% formalin mix for 24 h, and then the brain was placed in 20% sucrose for another 24 h. The brains were frozen and sectioned (30 μm) in the coronal plane, stained with cresyl violet, coverslipped, and examined microscopically. Histological analyses indicated that all recording sites were localized to the anterodorsal thalamus.
RESULTS
We recorded 14 HD cells from 6 animals in 13 sessions for experiment 1, and 30 HD cells from 5 animals in 18 sessions for experiment 2. One cell in experiment 1 was not tested in the Horizontal-Platform and Horizontal-Rotation sessions but was tested in the subsequent Vertical sessions. Two cells from two sessions in experiment 2 were only tested in the Horizontal-Platform and Horizontal-Rotation sessions but not the Vertical sessions, because cell isolation was lost before the first Vertical session; thus 16 sessions were used for the vertical wall experiments in experiment 2. In the Cylinder baseline session, the HD cells in experiment 1 had a mean peak firing rate of 43.57 ± 7.21 spikes/s, a directional firing range of 97.88 ± 5.45°, a background firing rate of 0.70 ± 0.25 spikes/s, and a Rayleigh r value of 0.834 ± 0.035. The HD cells used in experiment 2 had comparable values: mean peak firing rate = 41.30 ± 4.25 spikes/s; mean directional firing range = 110.18 ± 5.36°, background firing rate = 1.36 ± 0.28 spikes/s, and Rayleigh r value = 0.763 ± 0.023. Because the initial manipulations of the Horizontal-Platform and Horizontal-Rotation sessions were the same for both experiments, the results from the two experiments were combined. For some test sessions the isolation of the cell was lost before completion of all manipulations; therefore, there are different sample sizes for some sessions. Figure 3 shows representative responses from a HD cell undergoing the manipulations in experiment 1.
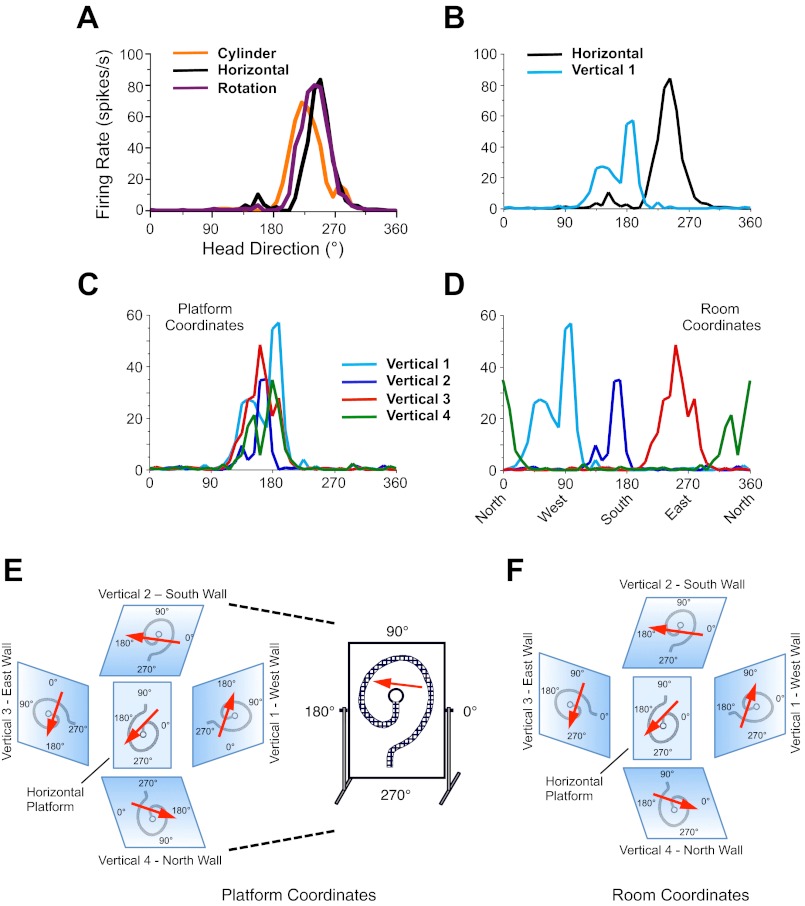
Fig. 3.
Representative tuning curves from a single head direction (HD) cell recorded in experiment 1. A: Cylinder, Horizontal-Platform, and Horizontal-Rotation sessions. B: Horizontal-Platform and Vertical 1 sessions. C: Vertical 1–4 sessions using the vertical platform as a reference frame. D: Vertical 1–4 sessions using the room as a reference frame. For this example, the cell's preferred direction was similar for the Cylinder and Horizontal-Platform sessions and did not shift after rotation of the horizontal platform (A). The cell's preferred direction was relatively similar for all Vertical sessions when using the vertical platform as the reference frame (C). The axis labels for all plots are shown in A. For purposes of clarity when comparing different sessions, note that the y-axis is drawn at a different scale in A and B compared with C and D. E and F: summary of HD cell responses for the Vertical sessions as a function of the reference frame used by the cell: platform coordinates (local cues) (E) correspond to C, room coordinates (global cues) (F) correspond to D. Note the different angular labels for the x- and y-axes when comparing the 2 coordinate systems.
Cylinder to Horizontal-Platform
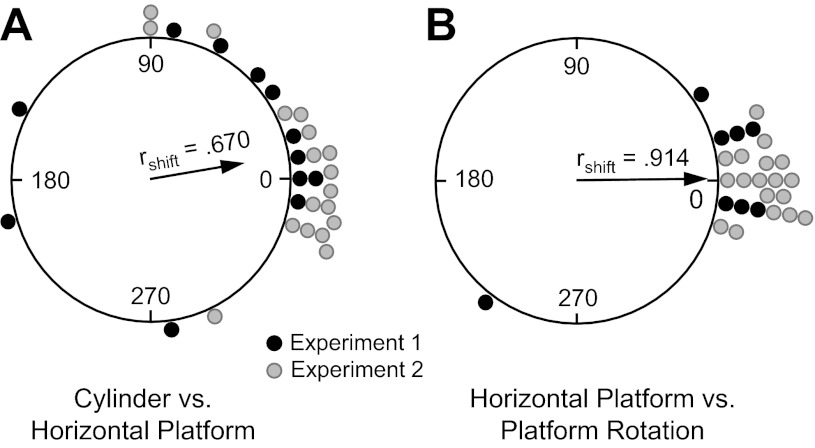
When the animal was transported from the holding cage to the raised horizontal platform after the Cylinder session, the preferred firing direction shifted to a new orientation (>27°) in 11 of 30 sessions (36.7%). Figure 4A is a circular histogram plotting the frequency of different shifts. Although there was a small tendency for shifts to occur in the CCW direction, a V-test showed that the shifts were significantly clustered around 0° [u = 3.57, n = 30, P < 0.001; mean shift Ø = 12.3 ± 46.5°].
Fig. 4.
Circular histograms showing the distribution of shifts in the preferred firing direction between the Cylinder and Horizontal-Platform sessions (A) and the Horizontal-Platform and Horizontal-Rotation sessions (B). The mean vector length (rshift) and Ø are shown for each distribution. Values are shown separated according to whether they were from experiment 1 or experiment 2.
For cells that did not have substantial shifts in their preferred direction, the results suggest that the cells used cues within the room as a reference frame (including the white cue card in the Cylinder sessions) for both the cylinder and horizontal platform environments (although the cue card was obviously absent for the Horizontal-Platform sessions). In contrast, for cells that shifted their preferred firing direction substantially when transferred to the horizontal platform, these cells apparently used a different set of cues within the room compared with when they were in the cylinder in the same room.
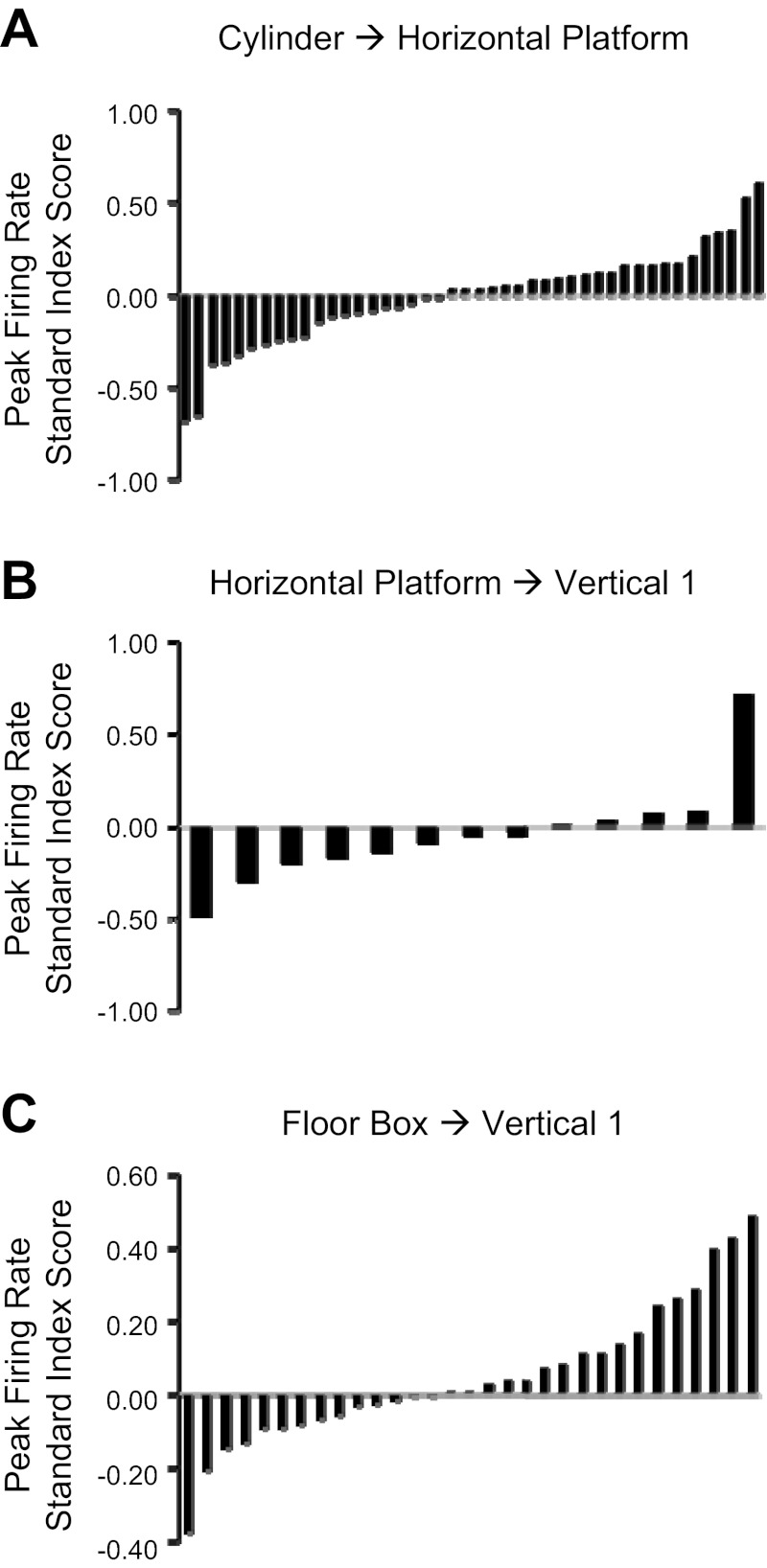
In contrast to the shifts in the cells' preferred firing directions, there was no change in the cells' peak firing rate, background firing rate, or Rayleigh r value between the Cylinder and Horizontal-Platform sessions. The mean standard index scores (±SE) were peak firing rateSI −0.007 ± 0.039 spikes/s, background firing rateSI 0.067 ± 0.058 spikes/s, Rayleigh rSI −0.034 ± 0.018. A t-test for each parameter revealed that these changes were not significantly different from the predicted value of 0 for no change (all P > 0.05). Plots showing the distribution of standard index scores were constructed for each parameter, and each plot showed a relatively uniform distribution of index scores (i.e., there was no bimodal distribution). Figure 5A depicts the distribution of standard index scores for peak firing rate. Thus, although the immediate environment of the animal changed significantly between the standard cylinder and the large horizontal platform, there was no evidence of any firing rate changes.
Fig. 5.
Standard index scores for peak firing rates between Cylinder → Horizontal-Platform sessions for experiments 1 and 2 (A), Horizontal-Platform → Vertical 1 sessions for experiment 1 (B), and Box 1 → Vertical 1 sessions for experiment 2 (C).
Horizontal-Rotation Sessions
When the horizontal platform was rotated ±90° or 180°, the preferred firing direction of almost all the neurons displayed only a small, if any, shift, suggesting that the cells were using cues along the walls and/or door of the recording room as a reference frame rather than the platform (for examples, see Fig. 3A and Fig. 8A). There were four cells in four sessions that were not tested in the Horizontal-Rotation session for experiment 1. Figure 4B depicts the frequency of different shifts between the Horizontal-Platform and Horizontal-Rotation sessions. Twenty-four of twenty-six sessions contained shifts of their preferred direction < 27°. A V-test indicated that there was not a significant shift in the preferred firing direction between the Horizontal-Platform and Horizontal-Rotation sessions, as the shifts between the two sessions were significantly clustered around 0° [u = 6.59, n = 26, P < 0.0001; mean shift Ø = 0.2 ± 23.8°]. This result indicates that when the rats were on the horizontal platform they usually used the room as a reference frame rather than the platform itself or local cues on the platform.
Fig. 8.
Representative tuning curves from a single HD cell recorded in experiment 2. A: Cylinder, Horizontal-Platform, Horizontal-Rotation sessions. B: Cylinder session compared with Box 1–4 sessions. C: Vertical 1–4 sessions using the vertical platform as a reference frame. D: Vertical 1–4 sessions using the room as a reference frame. For E–H, the Vertical sessions use the room as the reference frame. E: Box 1, Vertical 1 sessions. F: Box 2, Vertical 2 sessions. G: Box 3, Vertical 3 sessions. H: Box 4, Vertical 4 sessions. For this example, the cell's preferred direction was similar for the Cylinder and Horizontal-Platform sessions and did not shift after rotation of the horizontal platform (A). The cell's preferred direction was relatively similar for all Box sessions (B) and Vertical sessions when using the room as the reference frame (D). In the Vertical 2 session, the cell fired at 2 different directions as the animal traversed the spiral track—firing first at ∼35° and then firing again ∼15 s later along the track when the animal's head was pointing at ∼330° (see F, inset). This type of activity at 2 different directions during a vertical session occurred rarely. The axis labels for all plots are shown in A. For purposes of clarity when comparing different sessions, note that the y-axis is drawn at a different scale for the plots in B, F, and G compared with the plots in A, C, D, E, and H. I and J: summary of HD cell responses for the Vertical sessions as a function of the reference used by the cell: platform coordinates (local cues) (I) correspond to C, room coordinates (global cues) (J) correspond to D–H. For both I and J the ramps between the floor box and the vertical spiral track are not shown. Note the different angular labels for the x- and y-axes when comparing the 2 coordinate systems.
Experiment 1
Horizontal platform to vertical spiral track locomotion.
When the rats were placed at the beginning of the vertical spiral track and then locomoted along it to the center hole, all cells continued to fire in a direction-specific manner. That is, all cells displayed a single direction in which the cell preferred to fire. In general, firing in the vertical plane was indistinguishable from firing in the horizontal plane, and firing rate vs. HD tuning curves from vertical plane sessions looked similar to those recorded in the horizontal plane. There were no instances when a cell completely ceased firing when the rat was on the spiral track, and there was always only one direction in which the cell increased its rate of firing. For each HD cell there was little change in the cell's peak firing rate, background firing rate, or Rayleigh r value between the Horizontal-Platform and Vertical 1 sessions. The mean standard index scores for these three measures were peak firing rateSI −0.037 ± 0.075 spikes/s, background firing rateSI 0.167 ± 0.164 spikes/s, and Rayleigh r valueSI −0.073 ± 0.044. t-Tests revealed that these index scores were not significantly different from 0 (peak firing rate: t = −0.498, background firing rate: t = 1.023, Rayleigh r value: t = −1.642; all P > 0.05). Plots showing the distribution of standard index scores were constructed for each parameter, and each plot showed a relatively uniform distribution of index scores, without any evidence for a bimodal distribution. Figure 5B depicts the distribution of standard index scores for peak firing rate.
Previous studies have reported that HD cell responses on vertical surfaces are related to how the cells fire when the animal locomotes on a nearby horizontal surface (Calton and Taube 2005; Stackman et al. 2000). Specifically, these studies postulated that the reference frame on the vertical surface can be viewed within the three-dimensional (3D) coordinate frame of the room and the vertical plane coordinate system is simply a 90° rotation of the floor's x–y coordinate system into the vertical plane that the rat is situated in (Fig. 1). Thus, to further test this hypothesis, we compared each cell's preferred firing direction between the Horizontal-Platform and Vertical 1 sessions (e.g., Fig. 3B). However, given that the orientation of the tripod-mounted camera was different from the orientation of the ceiling camera (and thus was not based on room frame coordinates), we had to transform all directional headings obtained from the vertical plane in order for them to correspond to the same coordinate system as the ceiling camera (see methods). The amount of transformation depended on the wall on which the platform was positioned.
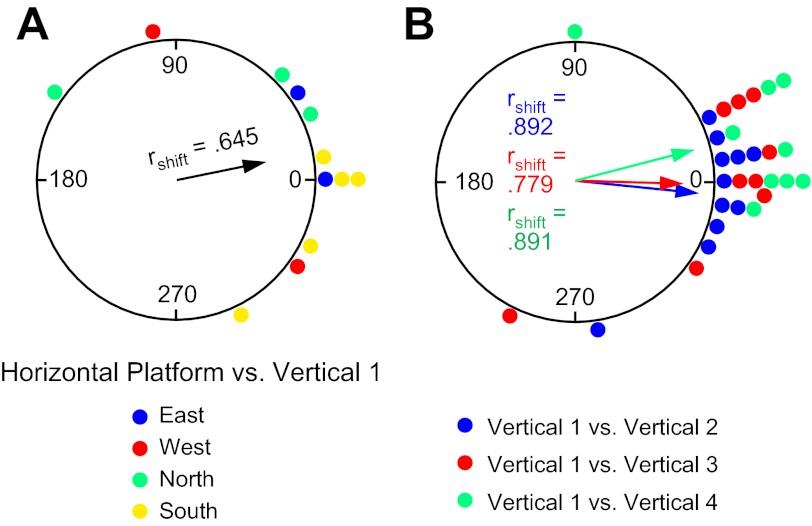
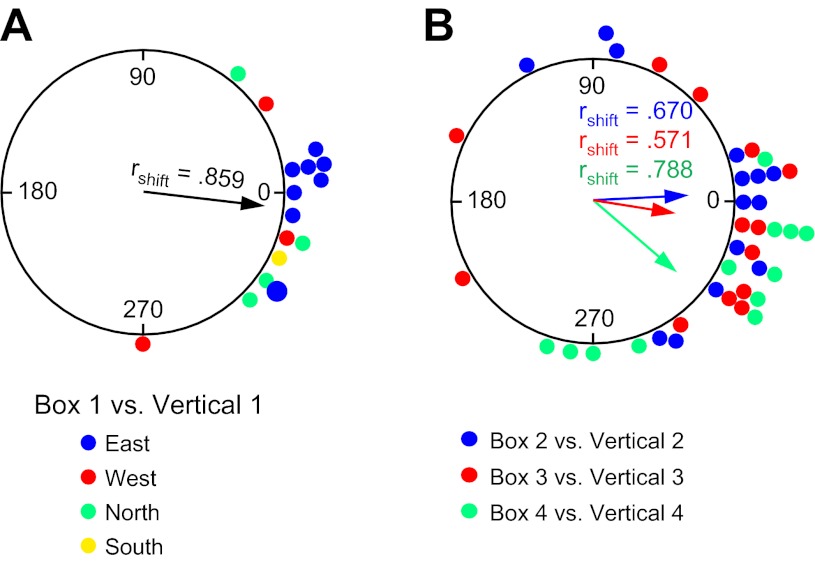
Figure 6A shows a circular histogram depicting the frequency of shifts in the cells' preferred firing directions between the Horizontal-Platform and Vertical 1 sessions after the necessary transformations of the vertical session coordinates. The mean shift across sessions (n = 12) was Ø = 10.6 ± 15.6°, rshift = 0.645. Based on how the coordinate frame for the vertical wall sessions is now defined, if cells continued to use the room as a reference frame (and simply rotated their horizontal plane of motion by 90° to the vertical plane), then the shifts for the Vertical 1 sessions should be near 0°. Figure 6A shows that the distribution of shifts is loosely clustered around 0°, with 9 of 12 sessions demonstrating shifts ≤45°. A V-test revealed a significant effect for clustering around 0° [u = 3.10, n = 12, P < 0.05], although an F-test on the parameters of concentration showed that the clustering was not as tight as the shifts between the Horizontal Platform and Horizontal Rotation sessions [F(1,40) = 4.33, P < 0.05]. Perhaps the increased variability in the shifts occurs because the rats have a different perspective of the room when locomoting on the vertical platform compared with the room viewed from the horizontal planes of the cylinder floor and horizontal platform. The clustering of shifts around 0° was independent of 1) which cardinal position the spiral platform was initially located at within the room (see Fig. 6A) and 2) the orientation of the rectangular platform during the prior Horizontal-Rotation session. In sum, these results indicate that, in general, the HD cells continued to use the room as a reference frame when the animals were placed on the vertically positioned platform, and are consistent with previous hypotheses (Calton and Taube 2005; Stackman et al. 2000) suggesting that when locomoting in the vertical plane animals extend the floor's horizontal coordinate system to the vertical wall surface through a 90° rotation of the horizontal coordinate system (Fig. 1).
Fig. 6.
Experiment 1. A: circular histogram showing the distribution of shifts in preferred firing direction between the Horizontal-Platform and Vertical 1 sessions. Color denotes which wall the vertical platform was located along for the Vertical 1 session. B: circular histogram showing the distribution of shifts in preferred firing direction between the Vertical 1 session and each of the subsequent Vertical 2–4 sessions. The mean vector length (rshift) and Ø are shown for each distribution.
Vertical platform positioned at the four cardinal locations.
To further test how an animal defines its spatial reference frame when locomoting in the vertical plane, we moved the vertical platform to each of the other three cardinal positions in the room (Fig. 2B, 5–7) and monitored HD cell responses (Verticals 2–4). All HD cells continued to fire in a direction-specific manner on the vertical spiral track at each of the other cardinal positions, with no observable changes in cell firing properties (Rayleigh r value, peak firing rate, background firing rate). Interestingly, however, their preferred firing directions were no longer aligned to room coordinates, but rather appeared to adopt the vertical platform itself as the reference frame. This result can most easily be seen by comparing the shift in the preferred firing direction between each of the Vertical sessions using the coordinate frame of the platform rather than the room's coordinate system (see Fig. 3, B–D). Since the tripod-mounted video camera always faced the vertical platform from the same orientation no matter where the platform was located in the room, no transformation of the camera's coordinates was necessary for this comparison. If the rat shifted its reference frame to the vertical platform when performing the spiral task, then one would expect minimal differences in the cells' preferred direction across the Vertical sessions at the different cardinal positions.
Fig. 7.
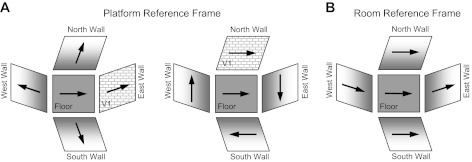
HD cell with a preferred firing direction pointing east on the floor and how it would hypothetically respond on the N, S, E, and W vertical walls if it used the vertical platform as a reference frame (as in experiment 1) and the Vertical 1 session (V1, depicted as a textured surface) started on the East wall (left) or the North wall (right) and the other walls served as the Vertical 2–4 sessions (A) or it used the room as a reference frame (as in experiment 2) (B). For the platform reference frame in A, note how the cell's preferred firing direction for the Vertical 1 session is aligned with the preferred firing direction on the floor, but thereafter in the Vertical 2–4 sessions the preferred firing direction is the same as the Vertical 1 session, but with respect to the platform—“up” for example on left and “right” for example on right. Thus, in platform coordinates, the preferred firing direction in Vertical 1 = Vertical 2 = Vertical 3 = Vertical 4. In contrast, in B, where the reference frame is the room, the preferred firing direction is different on each wall with respect to the platform, and the Vertical 1 session could occur along any of the 4 walls (note: floor box not shown).
Figure 6B plots the frequency of shifts in the preferred firing direction (in platform coordinates) between Vertical 1 and Vertical 2 (n = 11), between Vertical 1 and Vertical 3 (n = 9), and between Vertical 1 and Vertical 4 (n = 9). The mean Øs for the three comparison sessions were 355.1 ± 8.0°, 359.7 ± 12.7°, and 16.0 ± 8.9° for Verticals 2, 3, and 4, respectively, and were all significantly clustered around 0° [V-tests: Vertical 1-Vertical 2: u = 4.17, Vertical 1-Vertical 3: u = 3.31, Vertical 1-Vertical 4: u = 3.63; all P < 0.001]. The individual shifts are relatively small; indeed, 26 of 29 sessions (89.7%) had shifts ≤36° and are not significantly different from the shifts between Horizontal-Platform and Horizontal-Rotation when the cells appeared to use the room as a reference frame (Watson-Williams tests, F statistics, all P > 0.05). Overall, these results suggest that a reference frame based on the platform and spiral track was established during the first Vertical session and then used again during each of the following Vertical sessions at the other cardinal positions (see Fig. 7A for examples in which the Vertical 1 session would be along either the East or North walls, followed by Vertical 2–4 sessions at the other walls). This result therefore contrasts with those in the Horizontal-Platform and Horizontal-Rotation sessions where the room, and not the platform, was used as the reference frame. More importantly, this result also indicates that the reference frame in the vertical plane was not always a simple 90° transformation of the horizontal plane into the vertical plane and is not consistent with the predictions of the earlier studies (Calton and Taube 2005; Stackman et al. 2000). Note that in these earlier studies the animals were able to locomote freely between the horizontal and vertical planes, whereas in the present study the animals were passively moved by hand from their home cage to the start location on the spiral track. Thus it is possible that the observed discrepancy between the two studies occurred because the animals in experiment 1 were unable to locomote continuously between the horizontal and vertical surfaces—thus depriving them of self-motion cues between the two surfaces. Experiment 2 was designed to test this possibility.
Experiment 2
Experiment 2 was identical to experiment 1 except that for each of the Vertical sessions the animal was first placed into a small square box located on the floor at the base of the vertical platform, which contained a 45° ramp that led onto the vertical spiral track. Animals were recorded for 2 min in the square before being allowed to run up the ramp and onto the track; this session is referred to as the Box session, with numerals 1–4 indicating the corresponding subsequent vertical track session (i.e., Box 1-Vertical 1, Box 2-Vertical 2, etc.). Starting in the box enabled the animals to self-locomote from the horizontal surface (box floor) up the inclined ramp and onto the vertical platform surface. Once the rats entered the ramp incline they usually continued moving up the ramp, reaching the start of the vertical spiral track in 2–4 s. A total of 16 sessions (30 cells) were recorded from 5 animals for experiment 2; 1 of these 5 animals was initially used in experiment 1. Figure 8 shows representative responses from a HD cell undergoing the manipulations in experiment 2.
Box Sessions
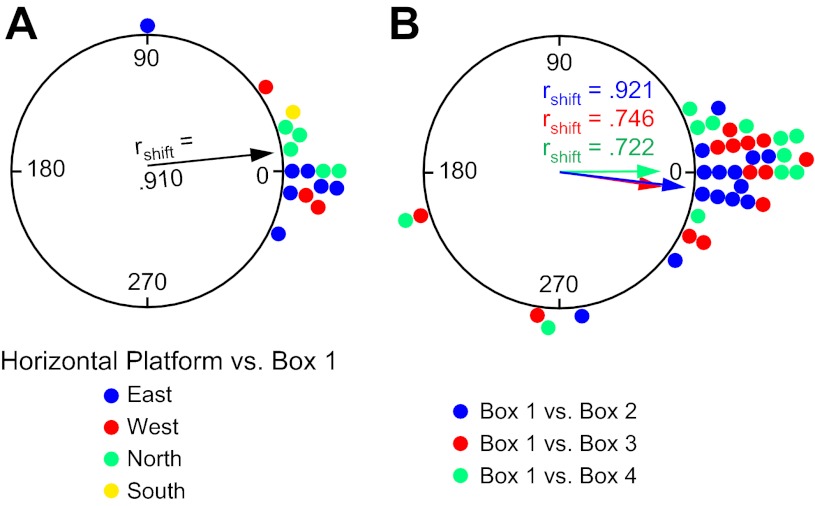
To determine the reference frame used by HD cells for the Box sessions, each cell's preferred firing direction in the Horizontal-Platform session was compared with its preferred firing direction in the Box/Vertical wall sessions with the room as the reference frame (defined by the overhead camera). The difference in the preferred firing direction between the Horizontal-Platform and Box 1 sessions (n = 16) was generally small, and the results are displayed in Fig. 9A, with different color points designating which cardinal wall the box was initially positioned along. The mean Ø was 6.2 ± 6.1° (rshift = 0.910); 15 of 16 sessions showed shifts ≤36°, and a V-test confirmed that the shifts were significantly clustered around 0° [u = 5.12, P < 0.0001]. These results indicate that the cells were most likely using room cues for their reference frame in both the Horizontal-Platform and Box 1 sessions.
Fig. 9.
Experiment 2. A: circular histogram showing the distribution of shifts in preferred firing direction between the Horizontal-Platform and Box 1 sessions. Color denotes which wall the box and vertical platform were located along for the Box 1/Vertical 1 session. B: circular histogram showing the distribution of shifts in preferred firing direction between the Box 1 session and each of the subsequent Box 2–4 sessions. The mean vector length (rshift) and Ø are shown for each distribution.
The preferred firing directions were also quite stable in subsequent Box sessions when the vertical platform was moved to the other cardinal positions (see Fig. 8B for an example). Figure 9B depicts the frequency of shifts in the preferred direction between Box 1 to Box 2 (n = 14), Box 1 to Box 3 (n = 13), and Box 1 to Box 4 (n = 12) sessions. The mean Øs for the three subsequent Box sessions were 352.8 ± 6.1°, 351.4 ± 11.3°, and 0.5 ± 12.3°, respectively, and did not differ from 0° [V-test u = 4.83, 3.76, 3.54; all P < 0.0001]. The clustering of points near zero for all Box sessions (34 of 39 sessions shifted ≤39°) indicates that the preferred directions were usually stable relative to the room, no matter which cardinal location the box and vertical platform were positioned, although there were five occasions on which this premise was not followed. [Interestingly, all five occasions came from two animals, and in each case the cells' preferred directions shifted with the box, suggesting that for these sessions the animals were using the box as a reference frame rather than the room.] Overall, these results provide strong evidence that the cells most frequently used a room coordinate system for locomotion during the Box sessions.
Box Sessions to Vertical Platform Locomotion
To compare the use of reference frames during transition between horizontal and vertical surfaces when motor efference/proprioceptive cues are available through self-locomotion, HD cells were monitored as the animal locomoted up the ramp onto the vertically positioned spiral track. Under these conditions and in contrast to the results from experiment 1, HD cells continued to use the room as a reference as opposed to the vertical platform as in experiment 1. In this way, the coordinate system in the floor box (the room reference frame) was extended to the vertical surface by rotating the coordinate system by 90° into the vertical plane along the animal's plane of motion (see Fig. 8E for an example). Figure 10A compares the amount of shift in the preferred firing direction between the Box 1 and Vertical 1 sessions (n = 16). If the cells' preferred directions on the vertical platform remained in alignment with the box session, then the shifts between the box session and vertical platform session should be clustered around 0°. For 14 of 16 sessions the cells' preferred directions shifted ≤45°. The overall mean vector for these 16 sessions was Ø = 354.2 ± 7.6° (rshift = 0.859); a V-test showed that these shifts were significantly clustered around 0° [u = 4.83, P < 0.0001]. This result was not dependent on the cardinal position of the first vertical platform session, as seven sessions started along the East wall, five sessions started along the North wall, three sessions started on the West wall, and one session started on the South wall. These results suggest that when self-motion cues are available the animal uses the room reference frame coordinates when on the floor and transfers this coordinate system by rotating it 90° onto the vertical wall, thus treating the wall's coordinate system as an extension of the floor's coordinate system.
Fig. 10.
Experiment 2. A: circular histogram showing the distribution of shifts in preferred firing direction between the Box 1 and Vertical 1 sessions. Color denotes which wall the vertical platform was located along for the Vertical 1 session. B: circular histogram showing the distribution of shifts in preferred firing direction for the Box 2 vs. Vertical 2, Box 3 vs. Vertical 3, and Box 4 vs. Vertical 4 sessions. The mean vector length (rshift) and Ø are shown for each distribution.
In general, there was no change in the robustness of the directional signal as measured by the Rayleigh scoreSI in locomoting from the box to the vertical platform (mean RayleighSI = −0.014 ± 0.014, t = −0.972, P > 0.05). In addition, there were no significant changes in the cell's peak firing rate (mean peak firing rateSI = 0.050 ± 0.033 spikes/s) or background firing rate (mean background firing rateSI = −0.115 ± 0.075 spikes/s) between box and vertical platform sessions. A t-test revealed that these changes were not significantly different from 0, the expected value for no change (peak firing rate: t = 1.505, background firing rate: t = −1.537; both P > 0.05). In addition, plots showing the distribution of standard index scores were constructed for each parameter, and each plot showed a relatively uniform distribution of index scores, without any evidence for a bimodal distribution. Figure 5C depicts the distribution of standard index scores for peak firing rate.
Vertical Platform Positioned at the Four Cardinal Locations
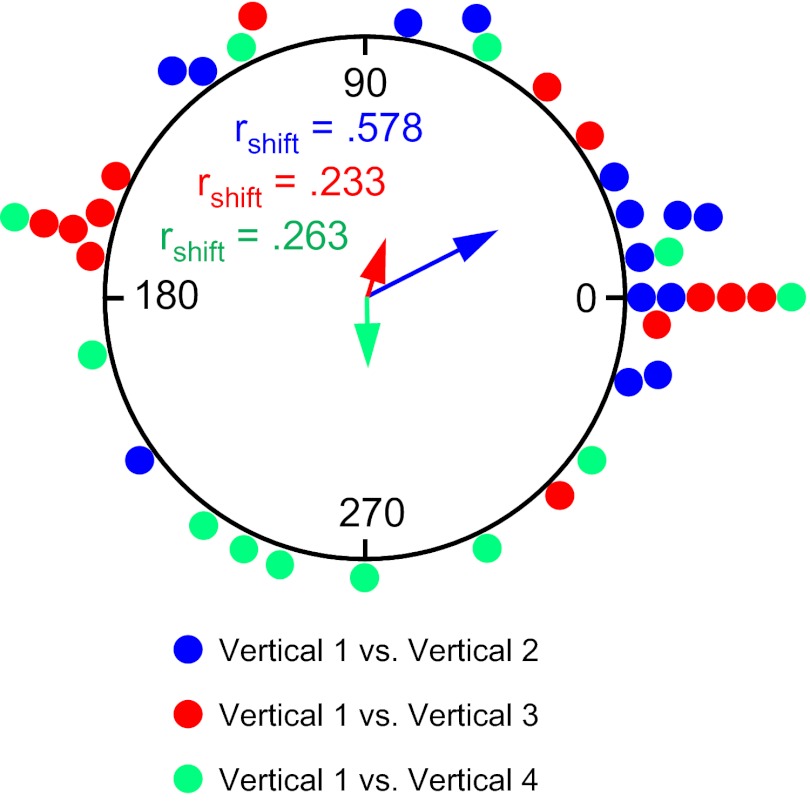
Each Vertical wall session for the three other cardinal positions was preceded by a 2-min session in the floor box. There was no attempt to disorient the animal between sessions. As with the Box 1 to Vertical 1 sessions there were no significant changes in any of the measured cell properties as the rat moved from the box onto the vertically positioned platform (all P > 0.05).
In terms of the cells' preferred firing direction, the pattern of results seen in the Box 1 to Vertical 1 sessions was usually repeated again in these subsequent sessions at the other cardinal positions. Thus when the rat moved from the box, up the ramp, and onto the vertical spiral track the cells' preferred directions usually remained in alignment with the box reference frame for that session (see Fig. 8, F–H for examples). Figure 7B shows an example of how a cell tuned to East would fire in the floor box and along each of the vertical wall surfaces in the room and can be contrasted to how the cell would have fired in the conditions for experiment 1. Figure 10B depicts circular histograms of the shifts in the preferred firing directions between the Box 2-Vertical 2 (n = 14), Box 3-Vertical 3 (n = 13), and Box 4-Vertical 4 (n = 12) sessions. The mean vector Øs for these histograms are 3.9 ± 12.4°, 351.2 ± 14.7°, and 320.1 ± 10.8°, respectively, and are all significantly clustered around 0° [V-test u = 3.54, 2.88, 2.96; all P < 0.005]. These Øs are similar to the Box 1 to Vertical 1 session (353.8°). However, the patterns of results observed in these subsequent Box to Vertical sessions were not as consistent as the Box 1 to Vertical 1 session. For example, the number of sessions that contained shifts ≤45° was 9 of 14 (64.2%), 8 of 13 (61.5%), and 8 of 12 (66.7%), for Box 2 to Vertical 2, Box 3 to Vertical 3, and Box 4 to Vertical 4 sessions, respectively—all percentages that were not as high as the Box 1 to Vertical 1 session (86.7%). This result is also reflected in the lower mean vector length values for these sessions (0.670, 0.571, 0.788) compared with the Box 1 to Vertical 1 session (0.849). Nonetheless, in the vast majority of sessions, the cells' responses reflected an extension of the reference frame in the box (the room reference frame) to the vertical surface by rotating the horizontal coordinate system by 90° into the vertical plane along the animal's plane of motion.
This change in how the cells defined their reference frame between experiments 1 and 2—from the reference frame of the vertical platform to the room reference frame—can best be appreciated when comparing the cells' preferred directions across vertical plane sessions (i.e., Vertical 1-Vertical 2, Vertical 1-Vertical 3, etc.) (also compare and note differences between Fig. 3, C and D, and Fig. 8, C and D). Recall that in experiment 1 the cells used the platform itself as a reference frame and there were no significant shifts in the cells' preferred directions when comparing Vertical 1 to Vertical 2, Vertical 1 to Vertical 3, and Vertical 1 to Vertical 4 (see Fig. 6B). In contrast, if the cells are not using the platform for their reference when in the vertical plane, then the shifts across Vertical sessions should vary considerably and not cluster around 0°, as they do in Fig. 6B. When this same analysis is applied to experiment 2, the shifts between Vertical 1 and the other Vertical sessions are much more distributed around the circle, as depicted in Fig. 11. The Øs for the mean vectors are not oriented around 0° for Vertical 1-Vertical 3 = 71.1 ± 19.7 (V-test: u = .385, n = 13, P > 0.05) and Vertical 1-Vertical 4 = 269.0 ± 20.1° (V-test: u = −0.021, n = 12, P > 0.05) but were for Vertical 1-Vertical 2 = 27.5 ± 14.1 (V-test: u = 2.71, n = 14, P < 0.05). However, there was a significantly greater dispersion in the shifts between Vertical 1-Vertical 2 sessions in experiment 2 than in experiment 1 [mean vector lengths: Vertical 1-Vertical 2: experiment 1 rshift = 0.892 vs. experiment 2 rshift = 0.578; F(7,11) = 5.75, P < 0.05]. The mean vector lengths for the other two comparisons in experiment 2 were also shorter than those in experiment 1 [Vertical 1-Vertical 3: experiment 1 rshift = 0.779 vs. experiment 2 rshift = 0.233, z score = 1.95, P = 0.051; Vertical 1-Vertical 4: experiment 1 rshift = 0.878 vs. experiment 2 rshift = 0.263, z score = 2.27, P = 0.023; both z score analyses corrected for 0.4 < rbar < 0.7 (Mardia 1972)]. In sum, these results indicate that when the animals self-locomoted into the vertical plane from the box via the ramp, the cells used the room as a reference frame throughout their passage and did not use the platform as a reference frame as they did in experiment 1.
Fig. 11.
Experiment 2. Circular histogram showing the distribution of shifts in preferred firing direction between the Vertical 1 session and each of the subsequent Vertical 2–4 sessions. The mean vector length (rshift) and Ø are shown for each distribution. The vertical platform was used as the reference frame (as opposed to room coordinates) for all Vertical sessions. Note the scattered distribution of points compared with Fig. 4B, indicating that the reference frame the animals used was not the platform—otherwise, the points would have clustered around zero.
DISCUSSION
The purpose of the present study was twofold: 1) to further evaluate how HD cells respond in the vertical plane and 2) to understand the nature of the reference frames used by HD cells as the animal transitions between the horizontal surface and the vertical plane. First, previous studies that monitored HD cell firing in the vertical plane primarily only sampled directional heading in two general directions 180° apart, and did not sample through an entire 360° (Stackman et al. 2000), making it difficult to construct accurate firing rate vs. HD tuning curves. Our results demonstrate that HD cell responses in the vertical plane, which were sampled over the entire 360° spectrum, are virtually identical to those seen in the horizontal plane, as the firing rate vs. HD tuning curves from the vertical plane were indistinguishable from those in the horizontal plane. Second, the cells used certain features of their environment for defining their reference frame (local vs. global cues); which features they used depended on how the animals moved between the two surfaces. If the animals were passively transported onto the vertical surface, then the cells usually used the vertical platform (local cues) as the reference frame. In this case the cells' preferred directions shifted with respect to the room cues as the vertical platform was moved to different locations within the room. In contrast, if the rats self-locomoted between the two planes, then the cells used the room reference frame (global cues), which the rat established in the floor box during its first experience there, and then rotated this coordinate frame by 90° through its plane of motion to extend it onto the vertical surface (Fig. 1).
Firing Properties in the Vertical Plane
HD cell firing properties were not affected by locomotion in the vertical plane. All cells maintained directional tuning when the rat was in the vertical plane, and activity was indistinguishable from cell activity when the rat was either on the floor in the cylinder or on the horizontally positioned platform. While place cells often undergo “rate remapping” after small changes in the environment or behavioral task contingencies (Leutgeb et al. 2005; Markus et al. 1995), there was no evidence that HD cells underwent any form of firing rate changes when the rats traversed the spiral track in the vertical plane. As discussed further below, there was also no evidence that cells' preferred directions remapped to new orientations when the rats moved into the vertical plane. Although the preferred directions sometimes shifted when the animals were placed or moved onto the vertical surface, the orientation they shifted to could be accounted for by the reference frame the cell was using, and in all cases when multiple cells were recorded simultaneously the cells' preferred directions remained “in register” with one another between the horizontal and vertical surfaces. Thus no “global remapping” occurred as is often seen in place cells between different environments (Bostock et al. 1991; Wills et al. 2005).
Is the HD Cell's Representation of Allocentric Space Inherently Two- or Three-Dimensional?
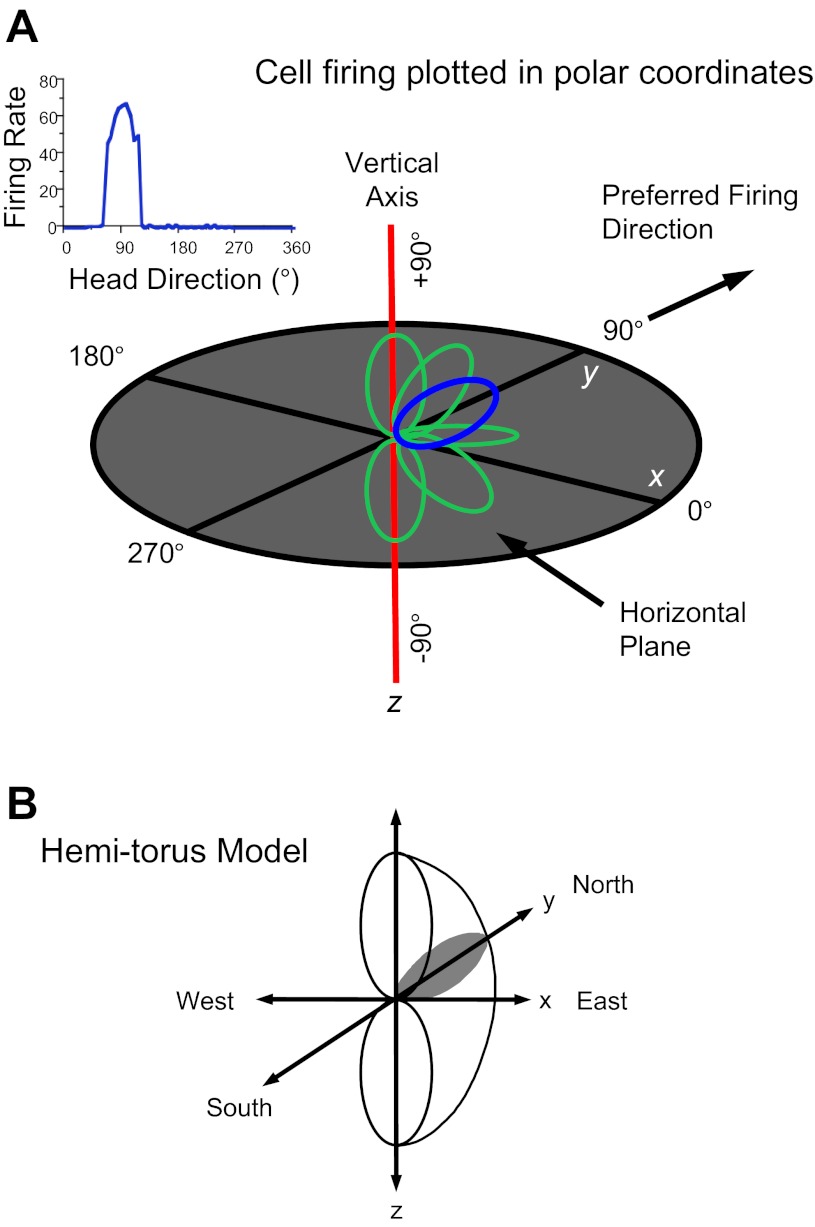
On the basis of previous studies we postulated that HD cell responses in three dimensions could be modeled based on the surface of a hemi-torus and referenced to the surrounding environment (Stackman et al. 2000). The model is constructed by plotting the cell's firing rate vs. HD tuning curve in polar coordinates and then rotating the polar plot around the vertical axis ±90° (Fig. 12). The model predicts that cell firing will cease abruptly as the animal pitches forward or backward >90° and for rolls >90° to the right or left. Previous findings showing that directional tuning is disrupted when an animal is inverted are consistent with this model (Calton and Taube 2005). The present findings demonstrating that cell firing is maintained in the vertical plane are also consistent with this model. There are two ways that one can imagine how the hemi-torus model might operate when a rat moves between 3D planes. One possibility is that the hemi-torus remains fixed in orientation with respect to the rat's head and when the rat's head rotates to another plane the hemi-torus rotates with it to maintain normal alignment with the rat's body axis. In this scenario, HD cells should maintain normal direction-specific firing as the rat moves about in any environment under any conditions of rotation and in any plane—including one that is inverted. However, the absence of directional activity when the rat is inverted argues against this view because the hemi-torus representation should continue to rotate with the rat as it moves into the inverted plane, and directional firing should still be observed.
Fig. 12.
Hemi-torus model for HD cell responses in 3 dimensions. A: HD cell tuning curve (top left) is plotted in polar coordinates in the horizontal x-y plane (blue trace). Pitching of the animal's head up or down along the vertical axis creates tuning curves outside the horizontal plane (green traces). Connecting all the polar coordinate plots for all possible orientations within ±90° creates the hemi-torus-shaped solid figure in B. The surface of this figure represents the cell's peak firing rate in 3 dimensions with respect to the global reference frame.
A second possibility is that the hemi-torus remains fixed in reference to the Earth's 3D coordinate frame. This view would be consistent with the observed disrupted directional signal when rats are inverted. It might also account for the observed striped/columnar firing patterns in grid cells when rats climbed up a vertical pegboard surface or locomoted along a spiral helix (Hayman et al. 2011). The absence of a well-defined 3D grid pattern was interpreted by the authors as evidence that the animals lacked a 3D volumetric representation of space, and instead generally represented space as a planar sheet in two dimensions that could be rotated depending on the spatial requirements of the animal. While interesting, these findings unfortunately do not provide a definitive answer as to whether the brain's spatial representations are inherently two-dimensionally structured or whether the neural network is based more on a 3D representation. This is because the rats in the Hayman et al. study were not truly moving in a vertical plane, since the rats moved tangentially to the vertical surface with the sides of their bodies adjacent to the vertical platform when they climbed the pegs. Thus the rats were oriented with their torsos more aligned to the floor, or pitched upward a little relative to their hind legs, rather than aligned to the vertical surface as they climbed the pegs.
Knierim and McNaughton (2001) examined place cell activity on a rectangular surface that was positioned at a 45° tilt and found that cells partially remapped under these conditions, suggesting that location-specific firing was sensitive to manipulations of the animal's plane of locomotion. Analyses also showed that the cells' place fields were not completely bound either to the room reference frame or to the 2D rectangular surface that the rats locomoted on. Furthermore, as with the Hayman et al. study, there was no evidence to support the notion that individual place cells were explicitly tuned to three dimensions. Taken together, the place and grid cell studies provide no support for the view that the brain's spatial representation of the world is based on a 3D structure. On the other hand, if the planar 2D model is correct then one might expect HD cell firing to be less disrupted when the animal locomotes inverted, since the inverted ceiling should be defined as the animal's plane of locomotion. Which of these models (planar vs. 3D) is operative in the brain is ultimately an important issue, as it has implications for how the brain performs spatial computations. Although our results on the spiral track can be interpreted as support for either model, future studies will need to address this issue further.
Reference Frame Shifts—Experiment 1
In experiment 1 the cells' preferred directions did not shift very much between the Cylinder and Horizontal-Platform sessions, suggesting that the cells were using room cues between the Cylinder and Horizontal-Platform sessions to maintain similar preferred directions in both environments. Note, however, that the clustering is not as tight around 0° as it was for the platform rotation sessions (compare Fig. 4, A and B). This result might be attributed to the fact that the cells most likely used the cylinder's cue card for their reference frame during the Cylinder session, but then relied on other cues in the room for the Horizontal-Platform session. Because the cells may not have been using these other room cues for the Cylinder session, it is possible the animals were not as accurate in judging their directional heading when switching to the room cues. Once they switched to the room cues, though, the cells continued to rely on them during the next session when the horizontal platform was rotated, as the cells' preferred directions did not shift between Horizontal-Platform and Horizontal-Rotation sessions (Fig. 4B). In this case, the cells avoided using the platform as a reference frame and continued to use the room cues. The cells continued using the room cues for their reference frame for the first vertical session, as the directional shifts between the Horizontal-Platform session and the Vertical session remained clustered around 0° (Fig. 6A), although, again, with more variability than in the rotation sessions. This increased variability could be attributed to the unfamiliarity of using the room cues from a new perspective on the vertical wall. After this first Vertical session, however, the cells then relied on the platform for their reference frame in experiment 1, as the subsequent Vertical sessions at the different room locations usually showed that there was little shift in the cells' preferred directions (Vertical 1 compared with Verticals 2–4; Fig. 6B, Fig. 7A).
Why do the cells use the room cues and not the platform as a reference frame for the first Vertical session (Vertical 1) but then switch and use the platform for the remaining Vertical sessions (Verticals 2–4)? It is possible that the familiarity of the platform in the vertical position was more salient than the use of surrounding room cues. The finding that cells switched reference frames—essentially from global cues to local cues, even within the same room and task—is neither surprising nor unusual. Other studies, all of which have been conducted entirely within the horizontal plane, have reported similar phenomena. For example, in experiments in which room and local cues were varied in the water maze task, Hamilton et al. (2007, 2008, 2009a) reported that rats initially used the room reference frame (distal cues) when introduced into the pool but thereafter switched and used the pool (local cues) during their swim to the hidden platform. The authors suggested that navigation in the water maze involved the use of a “movement vector” in which the distal room cues provide information about directional heading and the apparatus cues provide information about the distance of the goal (as well as the animal) from the pool wall. Similar findings with human subjects when tracking their gaze direction also found that they switched the set of cues they were using when performing a virtual reality water task (Hamilton et al. 2009b). Physiological studies with hippocampal place cells have also shown that cells can switch reference frames, even within the same recording session. Most of the switches occur between room-based and apparatus-based cues, where place cell discharge occurs either in relation to the room cues or in relation to local (apparatus) cues (Gothard et al. 1996; Knierim and Rao 2003; Shapiro et al. 1997; Siegel et al. 2007; Zinyuk et al. 2000).
Our results extend these place cell studies and show how HD cells can switch reference frames quickly, even when the global cues remain constant and clearly visible. However, in contrast to place cells, in which different subsets of place cells are organized in separate reference frames (Knierim 2002; Shapiro et al. 1997; Zinyuk et al. 2000), our results support the view that the HD cell network (at least within the anterodorsal thalamus) remains bound to a single reference frame at any given moment, and that the HD cell population does not split into groups with some cells responding to one reference frame and others responding to a second reference frame (Yoganarasimha et al. 2006). Although multiple spatial reference frames can be maintained online simultaneously within the place cell network, it remains to be determined whether the HD network can maintain representations of two directional headings simultaneously. Nonetheless, it is easy to imagine situations in which a subject can represent two different directional headings at the same time. For example, a subject could perceive that he/she is facing a particular direction within a room (I'm facing toward the front of the house) as well as the direction he/she is facing in relation to the larger-scaled environment (I'm facing toward the northeast side of town). One possibility would be to have different brain areas represent different environments that are at different scales (room vs. town). Recording simultaneously from multiple brain areas that contain HD cells could address this issue.
Reference Frame Shifts—Experiment 2
The only difference between experiments 1 and 2 was how the rats arrived at the vertical surface. Thus the reference frame used for the Cylinder and Horizontal-Platform sessions for experiment 2 was the same as in experiment 1. Likewise, the cells also used the room reference frame for the first Vertical session, but when the rat actively locomoted onto the vertical surface for the following Vertical sessions (2–4), the cells continued to use the room reference frame (global cues) throughout all the Vertical sessions (Fig. 11). Thus, unlike experiment 1, the cells did not switch and use the platform in the vertical position as their reference frame for the Vertical 2–4 sessions (compare Fig. 7, A and B, to contrast the two conditions). Apparently, the act of self-locomotion was particularly salient and the cells maintained their use of the room reference frame when the rats moved onto the vertical platform via the ramp. Thus under the right conditions—in this case self-locomotion—HD cells will consistently use the global room cues over the surrounding local cues for their reference frame (Zugaro et al. 2001).
Reference Frame Selection
It is worth considering the frames of reference used by the cells in the context of the two experiments. For both experiments, the rats were stationed in a nearby holding cage positioned on the floor before being placed into the particular apparatus. Thus it is possible that the animals could have “carried” the reference frame they used in their holding cage either onto the vertical platform (experiment 1) or into the floor box (experiment 2); in other words, their last experience before being placed into the experimental apparatuses could have determined the reference frame selected by the animals/cells. While this view can explain the results of experiment 2, it has difficulty accounting for the results in experiment 1, where the cells' preferred firing directions remained fixed to the platform for each of the Vertical sessions (Fig. 6B). According to the view that the last experienced reference served as the reference frame for the following Vertical sessions, the cells should have used the reference frame they used when the rats were in the holding cage prior to placement on the vertical platform. While we did not routinely monitor HD cell responses when the rats were in the holding cage, we could hear the cell fire from a loudspeaker in the adjacent room. These casual observations showed that the cells' preferred firing directions remained aligned either to the room (global cues) or to the holding cage itself across sessions. If either of these reference frames was aligned with the platform reference frame, then the cells' preferred firing directions should not have adopted the reference frame of the vertical platform, as they did for Vertical sessions 2, 3, and 4. Thus there was little evidence to suggest that the cells' preferred firing directions were aligned to the reference frame of either the room or the holding cage for these vertical sessions.
On a final note, because the rats were always hand-carried between the holding box and the apparatus, it is likely that they perceived a discontinuity between the reference frames of the holding cage and the apparatus. This perceived discontinuity would likely have required the rats to reorient each time they were placed on the apparatus for both experiments. For experiment 1, as discussed above, it appeared that the cells loosely followed the room's reference frame for the first Vertical session (Fig. 6A), but thereafter the cells used the vertical platform as their reference frame and not the room (Vertical sessions 2, 3, 4), as the cells' preferred firing directions were stable from one Vertical session to the next relative to the vertical platform reference frame (Fig. 6B). This finding suggests that the rats found the vertical platform as the most salient structure to use as a reference frame—one that was apparently more dominant than the room's global frame (once they had experienced the vertical platform). For experiment 2, the cells usually used the room as the reference frame when the rats were placed in the floor box. It is likely that they continued to use the room as the reference frame when the rats locomoted up the ramp onto the vertical platform, no matter which position the vertical platform was located in, because there was no perceived discontinuity between floor box and the vertical platform. Thus the perceived discontinuity between the different environments may compel the animal to adopt a new reference frame when it enters a second (discontinuous) environment.
Summary
HD cells used different aspects of the animal's environment for their reference frame when animals were locomoting in the vertical plane. Which features the cells used—global cues from the recording room or local cues associated with the immediate surface the rats' bodies were in contact with—depended upon the rats' surroundings, as well as how they moved between the differently oriented surfaces. When surrounding room (global) cues were available, HD cells generally used the visual cues within the room when the rats locomoted on horizontal surfaces, no matter how the surfaces were oriented. When the rats were passively transported from the floor of a nearby holding cage and placed onto the vertical plane surface (experiment 1), HD cells switched and used the vertical platform (local cues) as their reference frame. However, when the rats were allowed to self-locomote from a horizontal surface onto the vertical platform (experiment 2), HD cells continued to use the room as their reference frame. These experiments provide further support for the important role that self-motion cues serve in selecting the reference frame used by HD cells, and possibly the animal, during navigation (Stackman et al. 2003; Yoder et al. 2011).
GRANTS
This work was supported through National Aeronautics and Space Administration Cooperative Agreement NCC9-58 with the National Space Biomedical Research Institute and National Institutes of Health Grants MH-48924, MH-01286, and NS-053907 to J. S. Taube.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.S.T., S.S.W., and S.Y.K. conception and design of research; J.S.T., S.S.W., S.Y.K., and R.J.F. analyzed data; J.S.T., S.S.W., S.Y.K., and R.J.F. interpreted results of experiments; J.S.T., S.S.W., S.Y.K., and R.J.F. prepared figures; J.S.T. and S.S.W. drafted manuscript; J.S.T. edited and revised manuscript; J.S.T. approved final version of manuscript; S.S.W., S.Y.K., and R.J.F. performed experiments.
ACKNOWLEDGMENTS
The authors thank Jennifer Rilling for assistance in conducting these experiments and Michael Shinder for comments on the manuscript.
Preliminary reports of some of this research appeared in Chapter 3 of Head Direction Cells and the Neural Mechanisms of Spatial Orientation (Taube 2005).
REFERENCES
- Batschelet E. Circular Statistics in Biology. New York: Academic, 1981 [Google Scholar]
- Bostock E, Muller RU, Kubie J. Experience-dependent modifications of hippocampal place cell firing. Hippocampus 1: 193–205, 1991 [DOI] [PubMed] [Google Scholar]
- Calton JL, Taube JS. Degradation of head direction cell activity during inverted locomotion. J Neurosci 25: 2420–2428, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdikman D, Whitlock JR, Tsao A, Fyhn M, Hafting T, Moser MB, Moser EI. Fragmentation of grid cell maps in a multicompartment environment. Nat Neurosci 12: 1325–1332, 2009 [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The Organization of Learning. Cambridge, MA: MIT Press, 1990 [Google Scholar]
- Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci 16: 823–835, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Johnson TE, Rice JP, Candelaria FT, Redhead ES. Evidence for a shift from place navigation to directional responding in one variant of the Morris water task. J Exp Psychol Anim Behav Process 35: 271–278, 2009a [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Johnson TE, Rice JP, Candelaria FT, Sutherland RJ, Weisend MP, Redhead ES. The relative influence of place and direction in the Morris water task. J Exp Psychol Anim Behav Process 34: 31–53, 2008 [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Weisend MP, Redhead ES, Sutherland RJ. How do room and apparatus cues control navigation in the Morris water task? Evidence for distinct contributions to a movement vector. J Exp Psychol Anim Behav Process 33: 100–114, 2007 [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Johnson TE, Redhead ES, Verney SP. Control of rodent and human spatial navigation by room and apparatus cues. Behav Processes 81: 154–169, 2009b [DOI] [PubMed] [Google Scholar]
- Hayman R, Verriotis MA, Jovalekic A, Fenton A, Jeffery KJ. Anisotropic encoding of three-dimensional space by place cells and grid cells. Nat Neurosci 14: 1182–1188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ. Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J Neurosci 22: 6254–6264, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, McNaughton BL. Hippocampal place-cell firing during movement in three-dimensional space. J Neurophysiol 85: 105–116, 2001 [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Rao G. Distal landmarks and hippocampal place cells: effects of relative translation versus rotation. Hippocampus 13: 604–617, 2003 [DOI] [PubMed] [Google Scholar]
- Kubie JL. A drivable bundle of microwires for collecting single-unit data from freely-moving rats. Physiol Behav 32: 115–118, 1984 [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science 309: 619–623, 2005 [DOI] [PubMed] [Google Scholar]
- Mardia KV. Statistics of Directional Data. New York: Academic, 1972, p. 158–162 [Google Scholar]
- Markus EJ, Qin YL, Leonard B, Skaggs WE, McNaughton BL, Barnes CA. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci 15: 7079–7094, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cell, grid cells, and the brain's spatial representation system. Annu Rev Neurosci 31: 69–89, 2008 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates( 4th ed.). San Diego, CA: Academic, 1998 [Google Scholar]
- Shapiro ML, Tanila H, Eichenbaum H. Cues that hippocampal place cells encode: dynamic and hierarchical representation of local and distal stimuli. Hippocampus 7: 624–642, 1997 [DOI] [PubMed] [Google Scholar]
- Sharp PE, Blair HT, Cho J. The anatomical and computational basis of the rat head-direction cell signal. Trends Neurosci 24: 289–294, 2001 [DOI] [PubMed] [Google Scholar]
- Siegel JJ, Neunuebel JP, Knierim JJ. Dominance of the proximal coordinate frame in determining the locations of hippocampal place cell activity during navigation. J Neurophysiol 99: 60–76, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Golob EJ, Bassett JP, Taube JS. Passive transport disrupts directional path integration by rat head direction cells. J Neurophysiol 90: 2862–2874, 2003 [DOI] [PubMed] [Google Scholar]
- Stackman RW, Tullman ML, Taube JS. Maintenance of rat head direction cell firing during locomotion in the vertical plane. J Neurophysiol 83: 393–405, 2000 [DOI] [PubMed] [Google Scholar]
- Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci 15: 70–86, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. Head direction cell activity: landmark control and responses in three dimensions. In: Head Direction Cells and the Neural Mechanisms of Spatial Orientation , edited by Wiener SI, Taube JS, Cambridge, MA: MIT Press, 2005, p. 45–67 [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci 30: 181–207, 2007 [DOI] [PubMed] [Google Scholar]
- Taube JS, Burton HL. Head direction cell activity monitored in a novel environment and during a cue conflict situation. J Neurophysiol 74: 1953–1971, 1995 [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10: 420–435, 1990a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci 10: 436–447, 1990b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Lever C, Cacucci F, Burgess N, O'Keefe J. Attractor dynamics in the hippocampal representation of the local environment. Science 308: 873–876, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Clark BJ, Brown JE, Lamia MV, Valerio S, Shinder ME, Taube JS. Both visual and idiothetic cues contribute to head direction cell stability during navigation along complex routes. J Neurophysiol 105: 2989–3001, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoganarasimha D, Yu X, Knierim JJ. Head direction cell representations maintain internal coherence during conflicting proximal and distal cue rotations: comparison with hippocampal place cells. J Neurosci 26: 622–631, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinyuk L, Kubik S, Kaminsky Y, Fenton AA, Bures J. Understanding hippocampal activity by using purposeful behavior: place navigation induces place cell discharge in both task-relevant and task-irrelevant spatial reference frames. Proc Natl Acad Sci USA 97: 3771–3776, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugaro MB, Berthoz A, Wiener SI. Background, but not foreground, spatial cues are taken as references for head direction responses by rat anterodorsal thalamus neurons. J Neurosci 21: RC154, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]