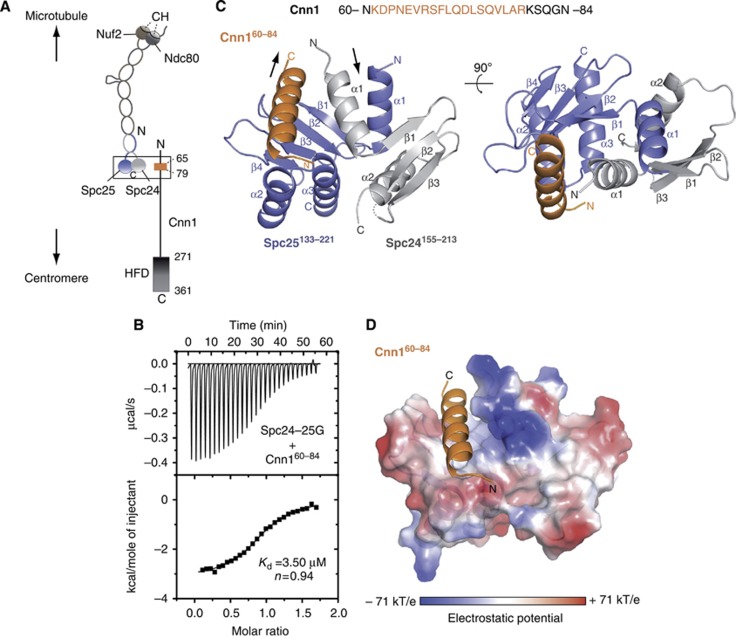
Figure 1.
Structure determination of the Spc24-25/Cnn1 interface. (A) Schematic representation illustrating the general centromere-microtubule orientation of the Cnn1–Ndc80 complex. Nuf2 and Ndc80 are shown in brown and dark grey, respectively, with the microtubule-binding calponin homology (CH) domains highlighted; Spc24 and Spc25 are shown in light grey and slate blue, respectively. The Cnn1 conserved N-terminal motif (65–79) is displayed in orange and its histone-fold domain (HFD) is highlighted. A rectangle indicates the interface visualized in the crystal structure. (B) Isothermal titration calorimetry performed by titrating the Spc24-25 globular domain (Spc24-25G) with a Cnn1 peptide including the Ndc80-binding motif (Cnn160–84). The Kd was estimated fitting a non-linear curve to the binding isotherms derived from the data. (C) Overall architecture of the Spc24155–213–Spc25133–221–Cnn160–84 crystal structure. Spc24, Spc25, and Cnn1 are represented according to their secondary structure and coloured in light grey, slate blue, and orange, respectively. Arrows indicate the opposite N-terminal/C-terminal orientation of Cnn1 relative to Spc24-25. A dotted line indicates the residue Lys184 of Spc24 not visible in the electron density (molecule B-A-F of the asymmetric unit). (D) Localization of Cnn160–84 in the hydrophobic pocket at the interface between Spc24155–213 and Spc25133–221, shown as electrostatic surface (transparency 20%). Cnn1 secondary structure is displayed in orange.