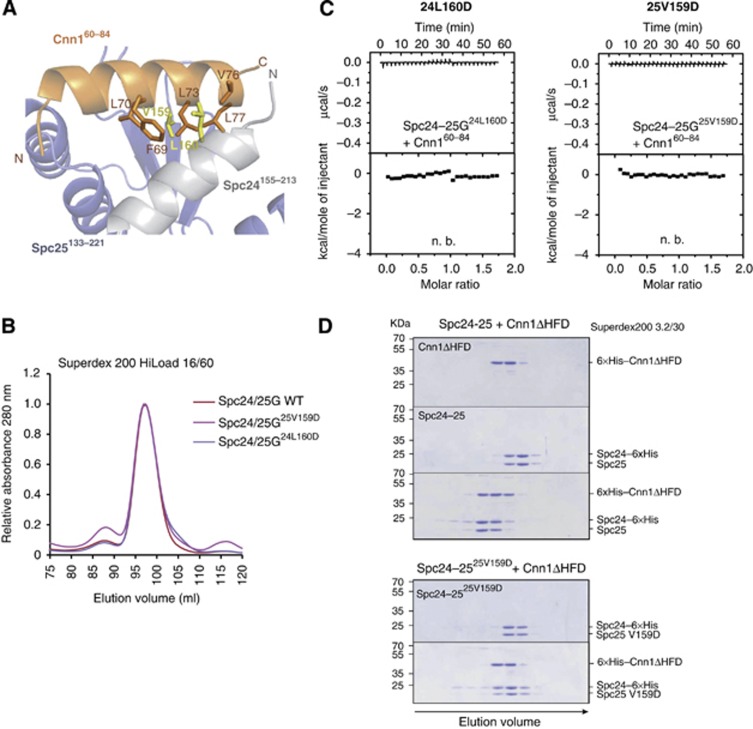
Figure 3.
Validation of the Spc24-25/Cnn1 interface. (A) Role of L160 (Spc24) and V159 (Spc25) in Cnn160–84 binding. The secondary structure of Spc24, Spc25, and Cnn1 is displayed in light grey, slate blue, and orange, respectively (transparency 40%). The residues L160 (Spc24) and V159 (Spc25), mutated to aspartic acid for validating the Spc24155–213–Spc25133–221/Cnn160–84 interface, are shown as sticks and highlighted in yellow. The Cnn1 residues that establish van der Waals contacts with L160 or V159 are displayed as sticks. (B) Elution profiles of size-exclusion chromatography performed with Spc24-25 globular domain (Spc24-25G) wild-type (in red) or Cnn1-binding mutants, Spc24-25G25V159D (in violet) and Spc24-25G24L160D (in magenta). Identical elution profiles indicate a similar complex shape and integrity. (C) Isothermal titration calorimetry performed by titrating Spc24-25G25V159D or Spc24-25G24L160D with Cnn160–84 (n.b.=no binding). (D) Coomassie-stained gels of analytical size-exclusion chromatography using 12 μM Cnn1ΔHFD (1–270), 12 μM full-length Spc24-25 wild-type and their combination (upper part); 12 μM full-length Spc25-25V159D and its combination in equimolar amount with Cnn1ΔHFD (lower part). Complex formation is visualized by a shift to earlier elution volumes of both molecules.