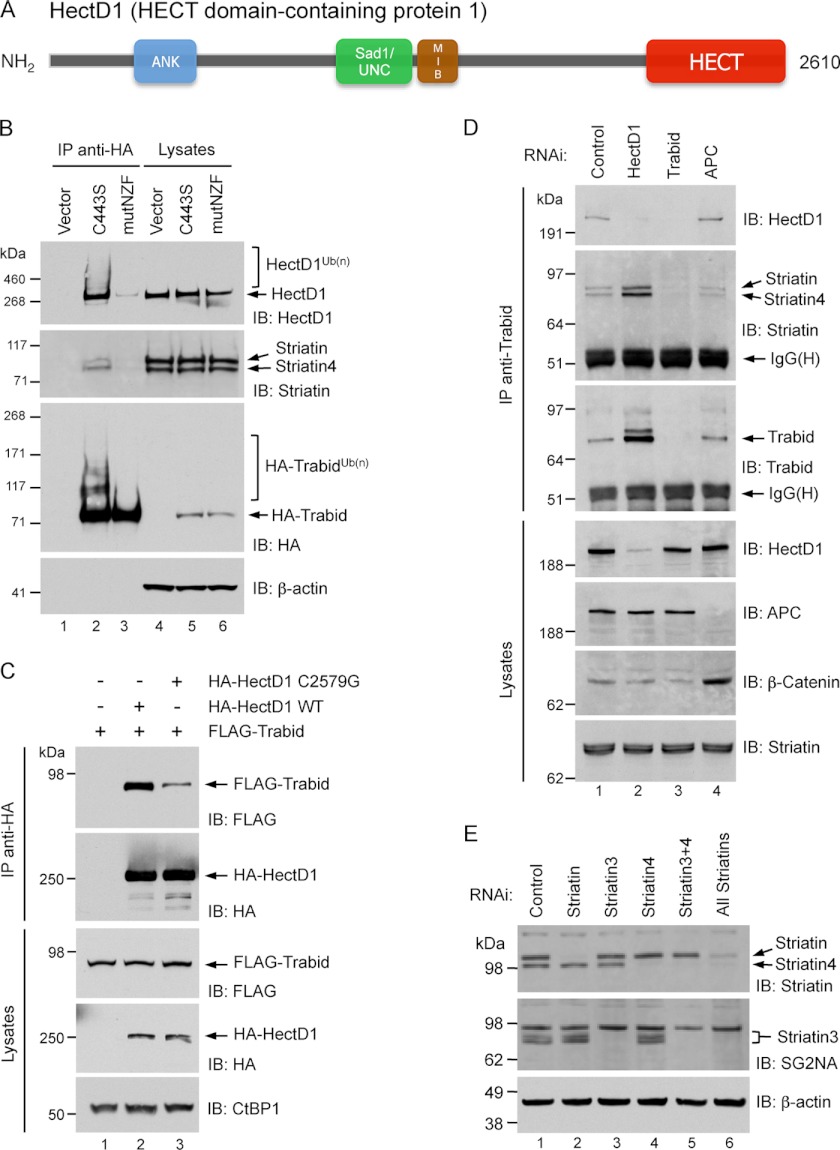
FIGURE 2.
A, domain structure of HectD1. ANK, ankyrin repeats. MIB, mind bomb. HECT, homologous to E6-AP C terminus. EMBL-EBI InterPro analysis predicted armadillo-like repeats encompassing the N-terminal third of HectD1. B, co-immunoprecipitation from transfected HEK293 cells between HA-Trabid C443S and endogenous HectD1 and Striatin. The cells were transfected with the indicated expression vectors for 48 h. The lysates were immunoprecipitated with anti-HA beads and analyzed by immunoblot (IB) as indicated. A Trabid C443S mutant that is also deficient in polyubiquitin-binding (mutNZF) exhibits minimal co-precipitation of HectD1 and Striatin. C, co-immunoprecipitation from transfected HEK293T cells between HA-HectD1 and FLAG-Trabid. The cells were co-transfected with the indicated expression vectors for 48 h. Lysates were immunoprecipitated with anti-HA beads and analyzed by immunoblot as indicated. D, co-immunoprecipitation from HEK293 cells between endogenous Trabid, HectD1, and Striatin. The cells were transfected with the indicated siRNAs for 72 h. The lysates were immunoprecipitated with anti-Trabid antibodies and analyzed by immunoblot as indicated. E, validating the specificity of siRNAs and antibodies to the three Striatin family members. HEK293 cells were transfected with the indicated siRNAs or their combinations (50 nm total) for 72 h. Lysates were analyzed by immunoblot with anti-Striatin (BD Biosciences; catalog no. 610838), which detects both Striatin and Striatin-4, as well as anti-SG2NA, clone S68 (Novus Biologicals), which is specific for Striatin-3 and detects several of its isoforms.