FIGURE 3.
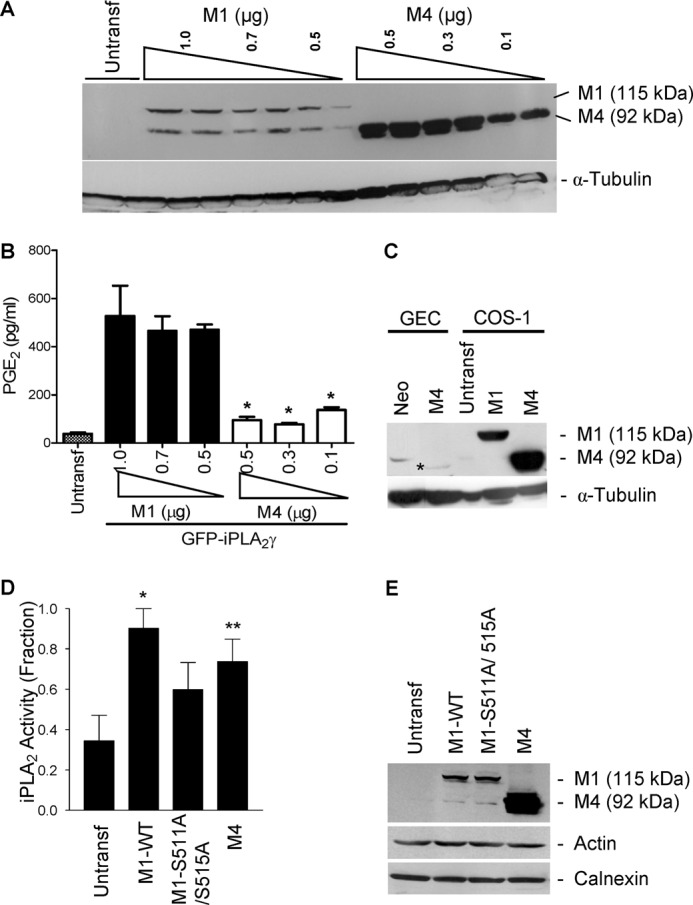
Expression and activity of GFP-iPLA2γ WT and mutants. COS-1 cells were co-transfected with N-terminally truncated (M4) GFP-iPLA2γ (0.1–0.5 μg of plasmid DNA) and for comparison with M1 GFP-iPLA2γ WT (0.5–1.0 μg of plasmid DNA), both with COX1. A, anti-GFP antibody immunoblot shows greater expression of M4 GFP-iPLA2γ compared with M1 GFP-iPLA2γ WT. Expression tended to increase with increasing doses of plasmid DNA. The lower band in the M1 lanes is nonspecific. B, PGE2 release in COS-1 cells expressing M4 GFP-iPLA2γ or M1 GFP-iPLA2γ WT (both with COX1) was normalized for corresponding protein expression. In these experiments basal PGE2 release (untransfected cells) was 38.2 pg/ml. PGE2 production by M1 GFP-iPLA2γ WT was markedly greater compared with M4 GFP-iPLA2γ. *, p < 0.0001 M4 + COX1 (0.5, 0.3, and 0.1 μg) versus M1 + COX1 (1, 0.7, and 0.5 μg), three experiments. C, GECs were stably transfected, and COS-1 cells were transiently transfected with M4 GFP-iPLA2γ. GEC-Neo and COS-1 cells transiently transfected with M1 GFP-iPLA2γ WT are presented for comparison. Lysates were immunoblotted with antibody to GFP. * denotes M4 GFP-iPLA2γ in GECs. The bands in GEC-Neo and untransfected COS-1 cells are nonspecific. D, PLA2 activity in untransfected (control) COS-1 cells and COS-1 cells expressing M1 GFP-iPLA2γ WT, M1 GFP-iPLA2γ S511A/S515A double mutant, and M4 GFP-iPLA2γ is shown. Cell extracts were prepared 24 h after transfection, and iPLA2 activity was monitored by release of AA from 2-arachidonoyl-phosphatidylcholine (“Experimental Procedures”). iPLA2 activities of M1 GFP-iPLA2γ WT and M4 GFP-iPLA2γ were significantly greater compared with control. iPLA2 activity of M1 GFP-iPLA2γ S511A/S515A tended to be greater than control. *, p < 0.01 M1 GFP-iPLA2γ WT versus control, **, p < 0.05 M4 GFP-iPLA2γ WT versus control, four experiments. In these experiments basal iPLA2 activity (control cells) was 0.69 nmol/min/ml. E, cell lysates were immunoblotted with antibodies to GFP (iPLA2γ), actin, or calnexin (marker of ER).
