FIGURE 3.
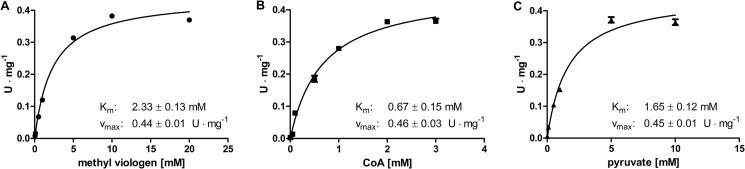
Kinetic parameters of recombinant C. reinhardtii PFR1 heterologously produced in E. coli and purified via His-tag affinity chromatography. Enzymatic activity was determined following the reduction of methyl viologen spectrophotometrically in 100-μl reaction mixtures containing 1.4 μm PFR1, 5 mm TPP, 16 mm dithioerythritol in 100 mm Tris-HCl, pH 8. The Km values of the individual substrates were determined in the presence of 2 mm CoA and 10 mm pyruvate (A, methyl viologen), 10 mm methyl viologen and 10 mm pyruvate (B, CoA), or 2 mm CoA and 10 mm methyl viologen (C, pyruvate). Each kinetic was analyzed from two independent PFR1 preparations as technical duplicates. The Km and Vmax values were calculated using GraphPad Prism® software. The graphs show the mean values, and the error bars indicate the standard deviation.
