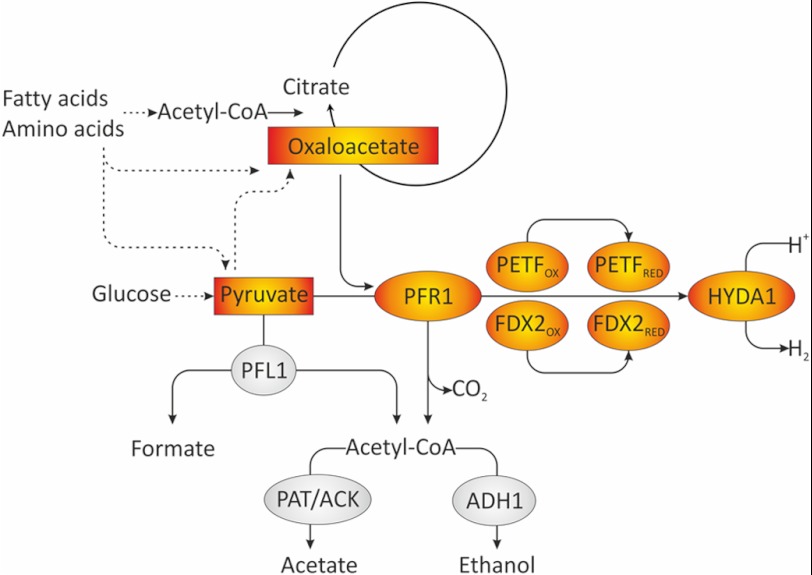
FIGURE 6.
Model of fermentative pathways involved in dark anaerobic H2 production in C. reinhardtii. In the wild type, PFL1 is the major fermentative enzyme in short term anaerobiosis and cleaves pyruvate into formate and acetyl-CoA. The latter can be reduced to ethanol via bifunctional acetaldehyde-alcohol dehydrogenase (ADH1) (92) or converted to acetate via phosphotransacetylase and acetate kinase (PAT/ACK). In addition to PFL1, PFR1 is capable of pyruvate oxidation. In a pfl1 mutant or in long term anaerobiosis, pyruvate oxidation might be preferably catalyzed by PFR1. PFR1 transfers electrons to ferredoxins and thereby allows H2 generation via the [Fe-Fe]-hydrogenase. In addition to pyruvate, PFR1 is able to use oxaloacetate as a substrate. This might link the oxidation of other substrates such as fatty acids or amino acids to fermentative H2 production, possibly via an anaerobically operating glyoxylate cycle or parts thereof.