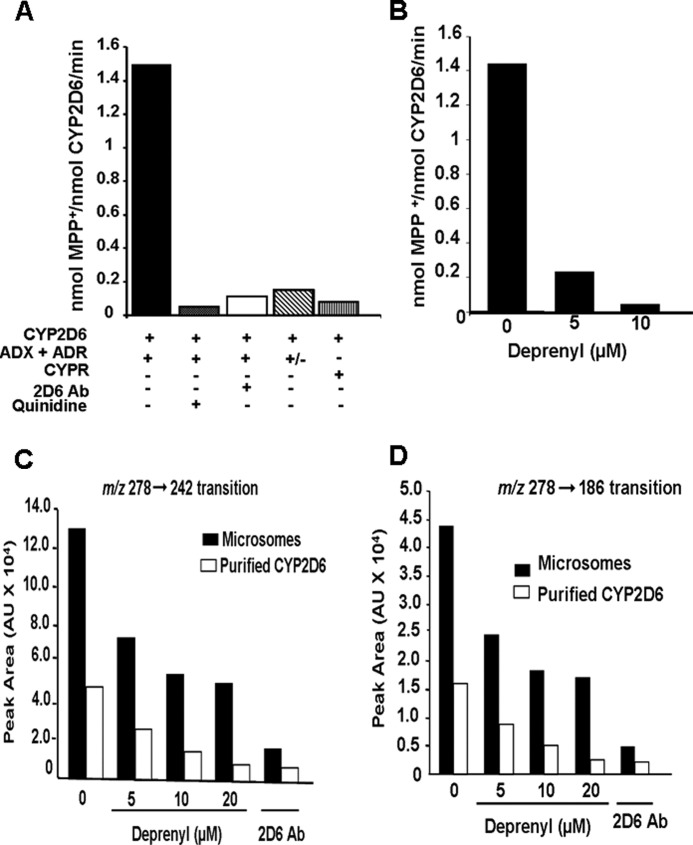
FIGURE 2.
Oxidation of MPTP and bufuralol by purified CYP2D6 reconstituted with Adx and AdxR. CYP2D6-purified enzyme was reconstituted with mitochondrial electron transfer proteins, Adx and AdxR, or microsomal electron transfer protein NPR in the presence or absence of added inhibitors as described under “Materials and Methods.” A, levels of MPP+ formed were quantified using an LC-MS method as described under “Materials and Methods.” The control sample in A was treated with 10 μl of ascites fluid (10 mg/ml), and the corresponding control without treatment with ascites is presented in B. In the indicated reactions, the CYP2D6 antibody was added at 10 μg/reaction, and quinidine was added at 10 μm. In the reaction marked “+/−”, Adr was omitted from the assay mixture. The value for MPP+ formed in each case represents the mean ± S.E. for three separate estimates. B, effects of increasing concentrations of deprenyl (5 and 10 μm) on MPTP metabolism was tested. In vitro reactions with purified CYP2D6 were run, and the MPP+ metabolite was quantified as described under “Materials and Methods.” C and D, microsomes from −SRP CYP2D6-expressing cells (200 μg each) and purified CYP2D6 reconstituted with Adx and Adr as in described in Fig. 1 and under “Materials and Methods” were used for bufuralol oxidation assays, and the products were subjected to LC/MS/MS analysis. The indicated concentrations of deprenyl were added to reactions. In one assay, 10 μg of CYP2D6 antibody was added. 1′-Hydroxybufuralol was monitored using two transitions, m/z 278 → 242 and m/z 278 → 186.