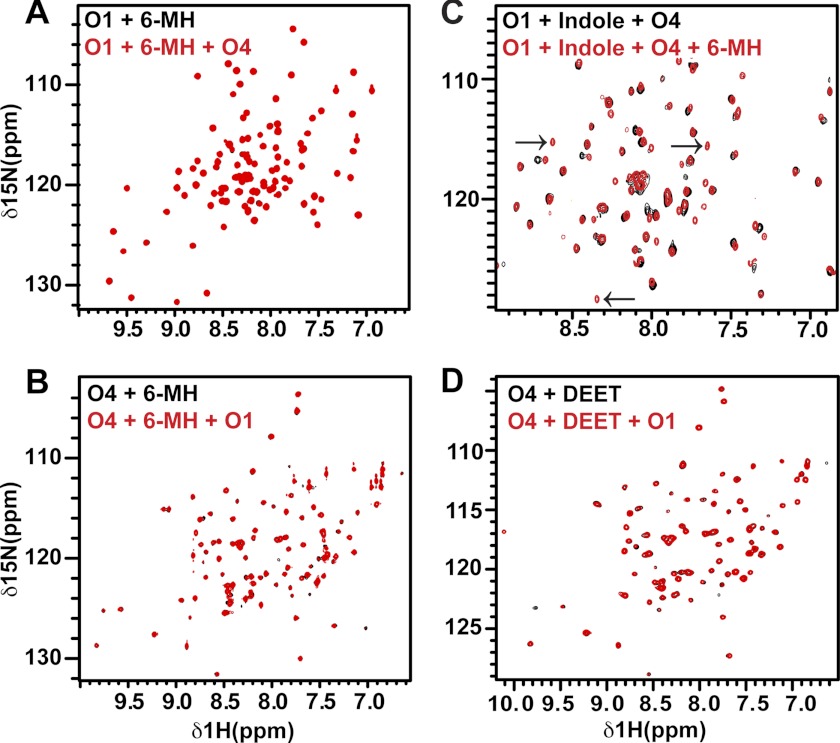
FIGURE 2.
There is no interaction between OBP1 and 4 in the presence of 6-MH. A, 1H-15N HSQC spectrum of 15N-OBP1 (O1) in the presence of 3 mm 6-MH (black) overlaid with the spectrum recorded in the presence of OBP4 (O4) (red). The spectra are essentially identical, consistent with a lack of interaction between OBP1 and OBP4. B, 1H-15N HSQC spectrum of 15N-OBP4 with 3 mm 6-MH (black). Addition of OBP1 (red) does not result in any chemical shift changes confirming the lack of interaction between the two proteins in the presence of 6-MH. C, 6-MH competing with indole for binding to OBP1 in the presence of OBP4. Spectrum of 15N-OBP1 plus indole plus OBP4 (black) overlaid with the spectrum of 15N-OBP1 plus indole plus OBP4 plus 6-MH (red) is shown. The black arrows indicate peaks only observed when OBP1 is bound to 6-MH. D, DEET inducing conformational ordering of OBP4 but not allowing interaction between OBP1 and 4. 1H-15N HSQC spectrum of OBP4 in the presence of DEET (black) overlaid with the spectrum of OBP4 plus DEET plus OBP1 (red) is shown. The lack of shifts indicates no interactions between the two proteins. All spectra were recorded in 20 mm sodium phosphate, pH 7.4, at 25 °C.