Abstract
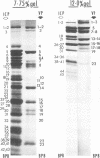
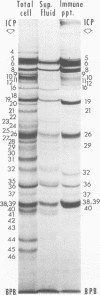
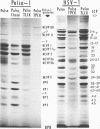
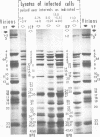
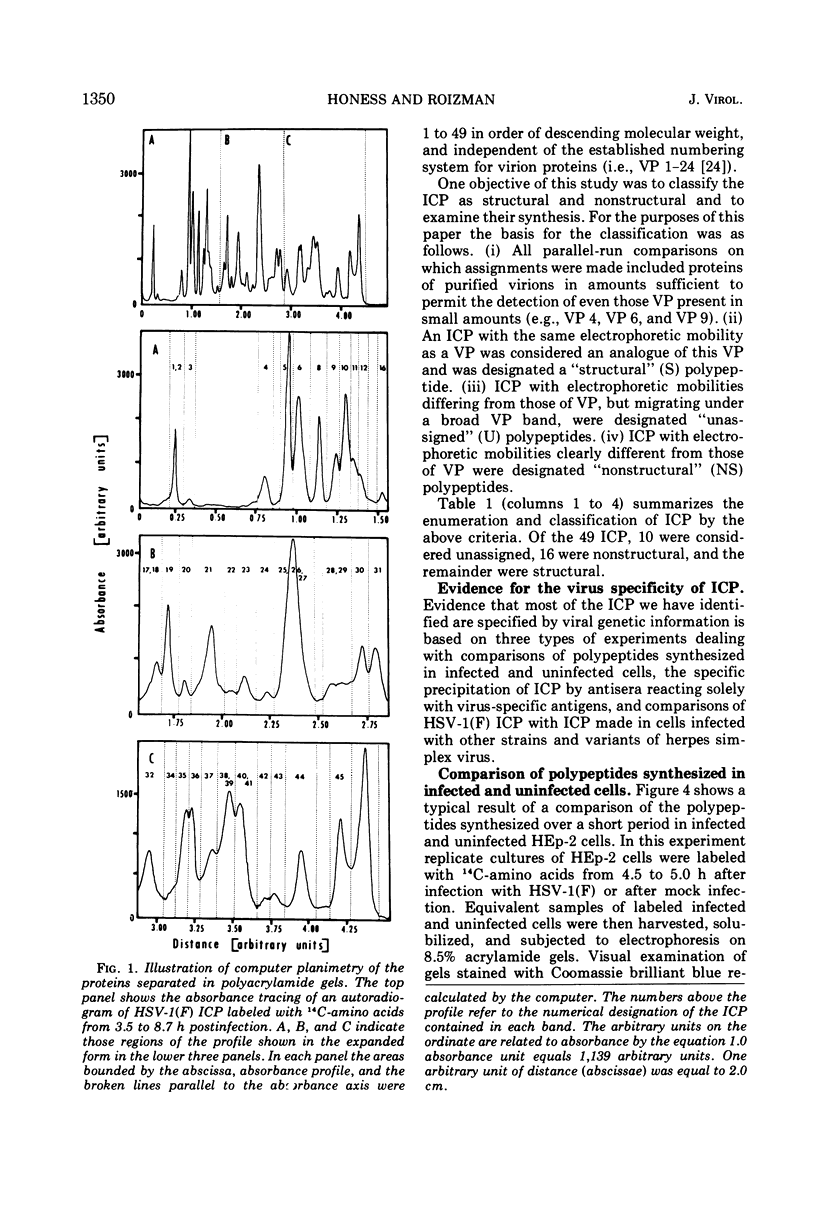
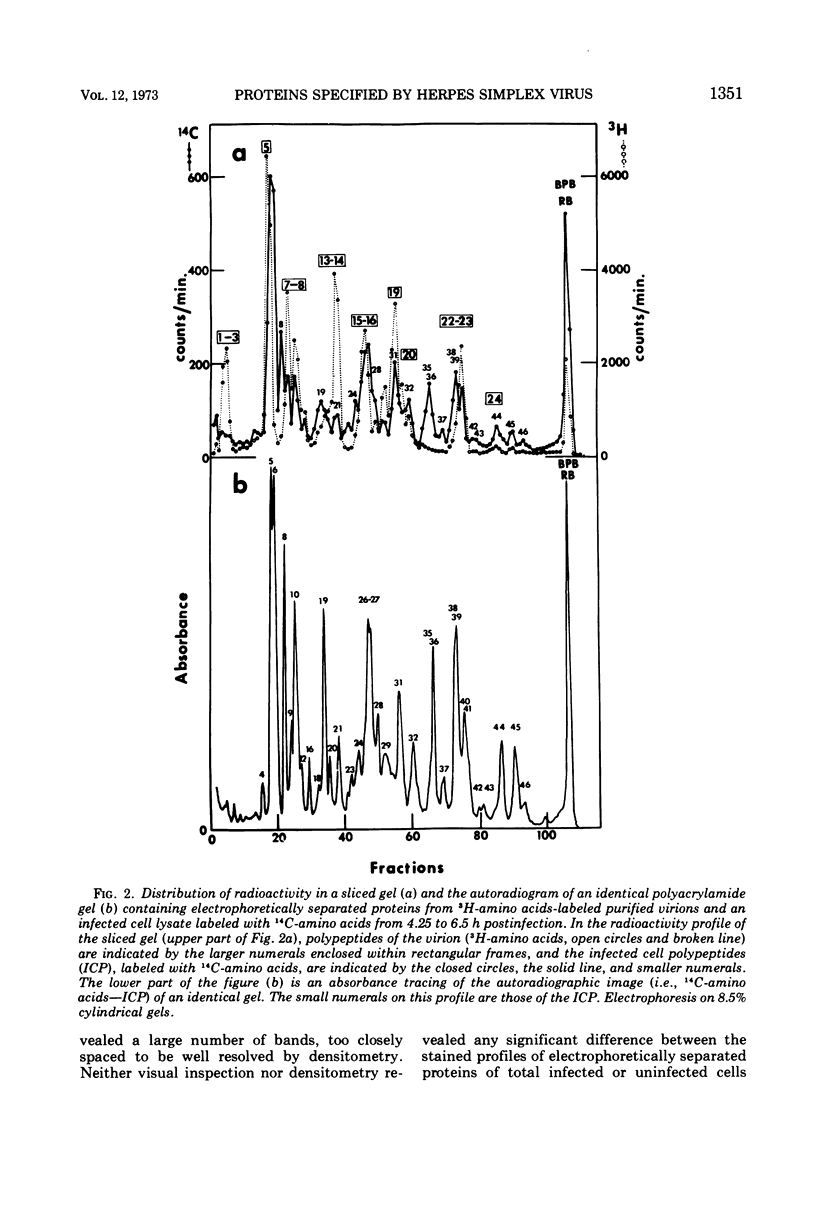
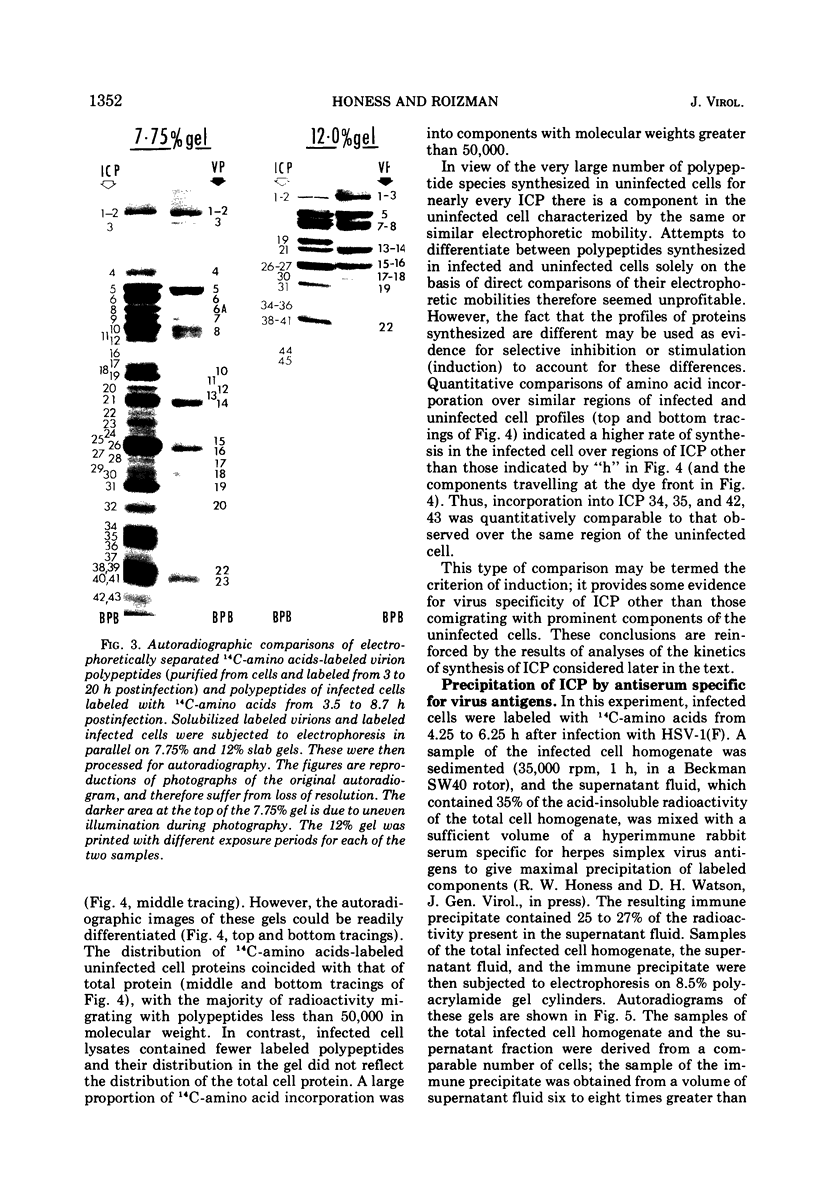
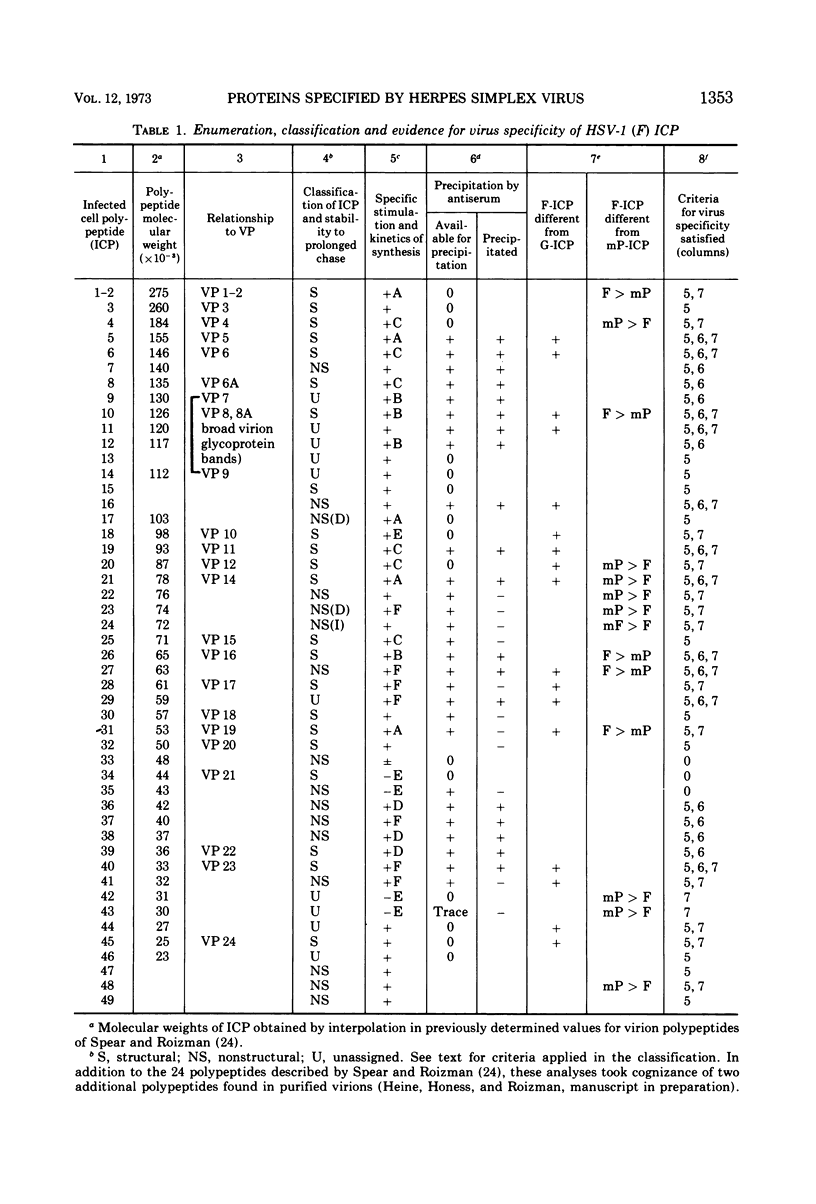
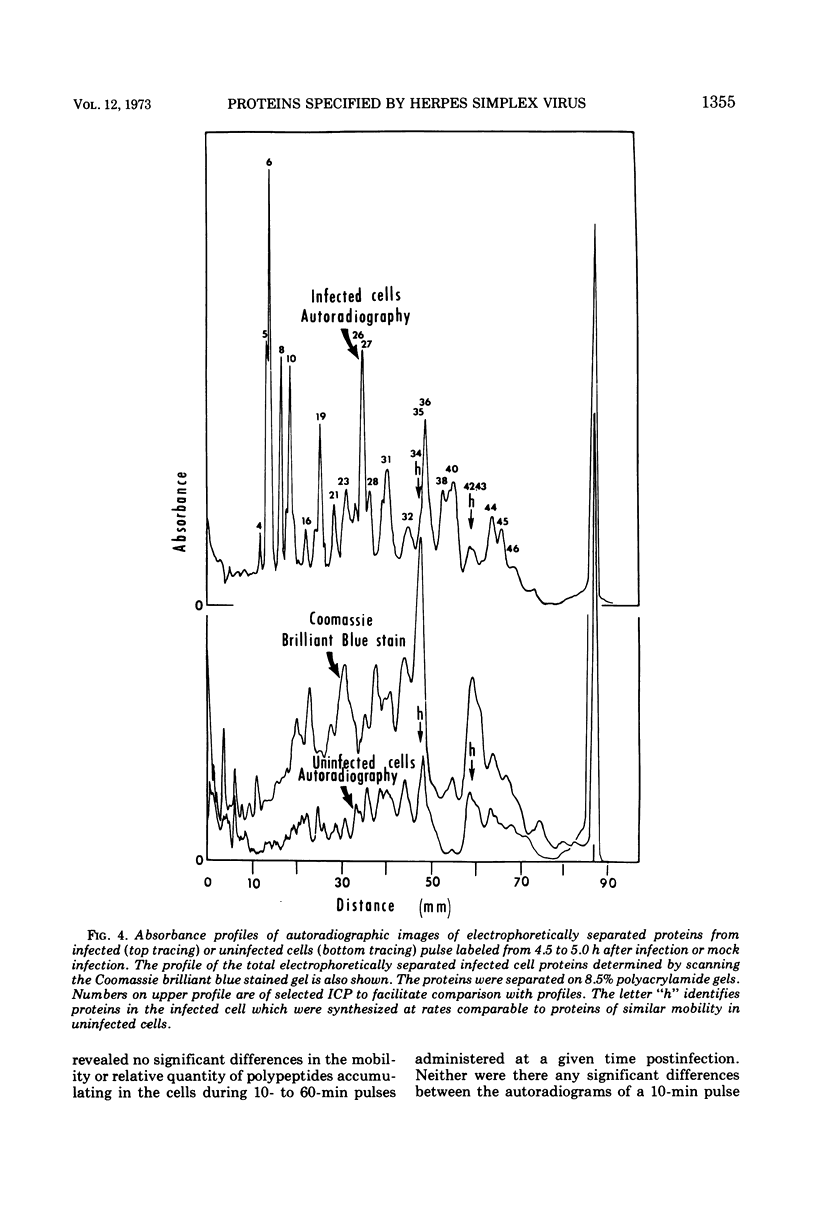
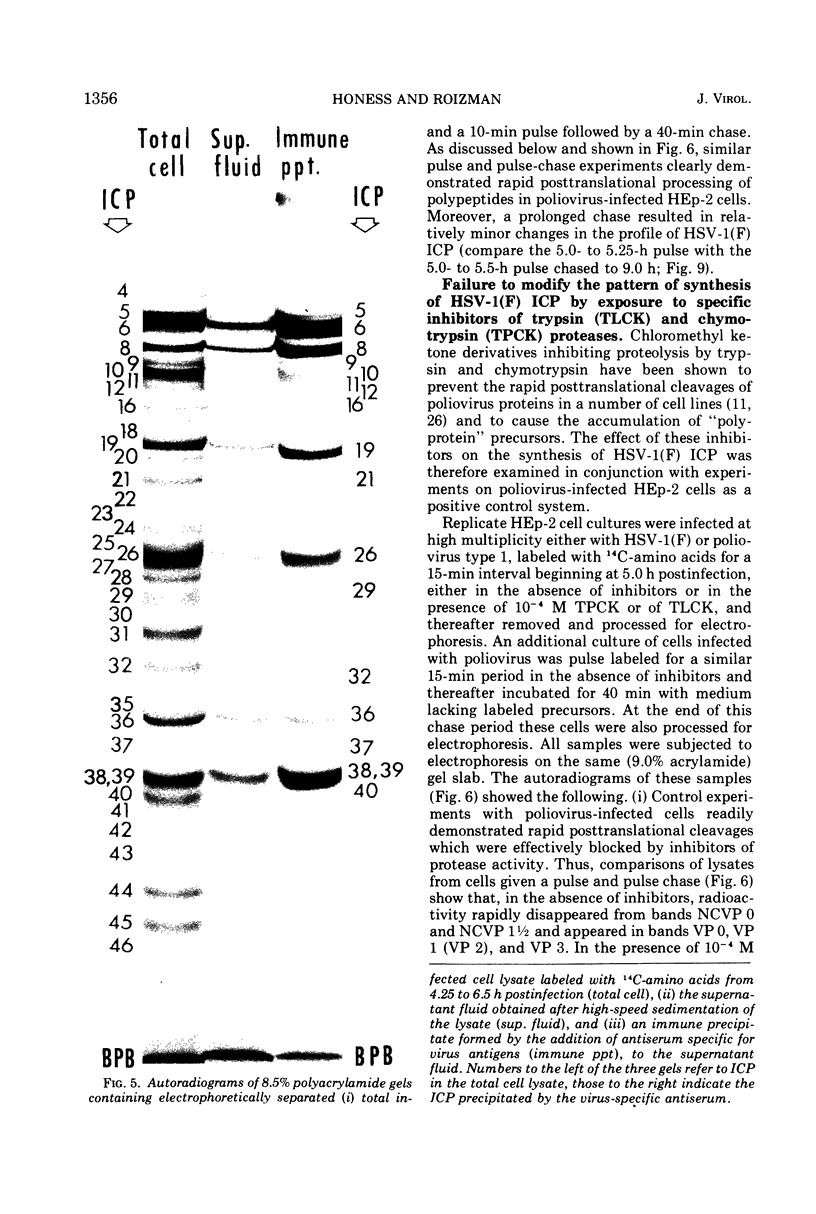
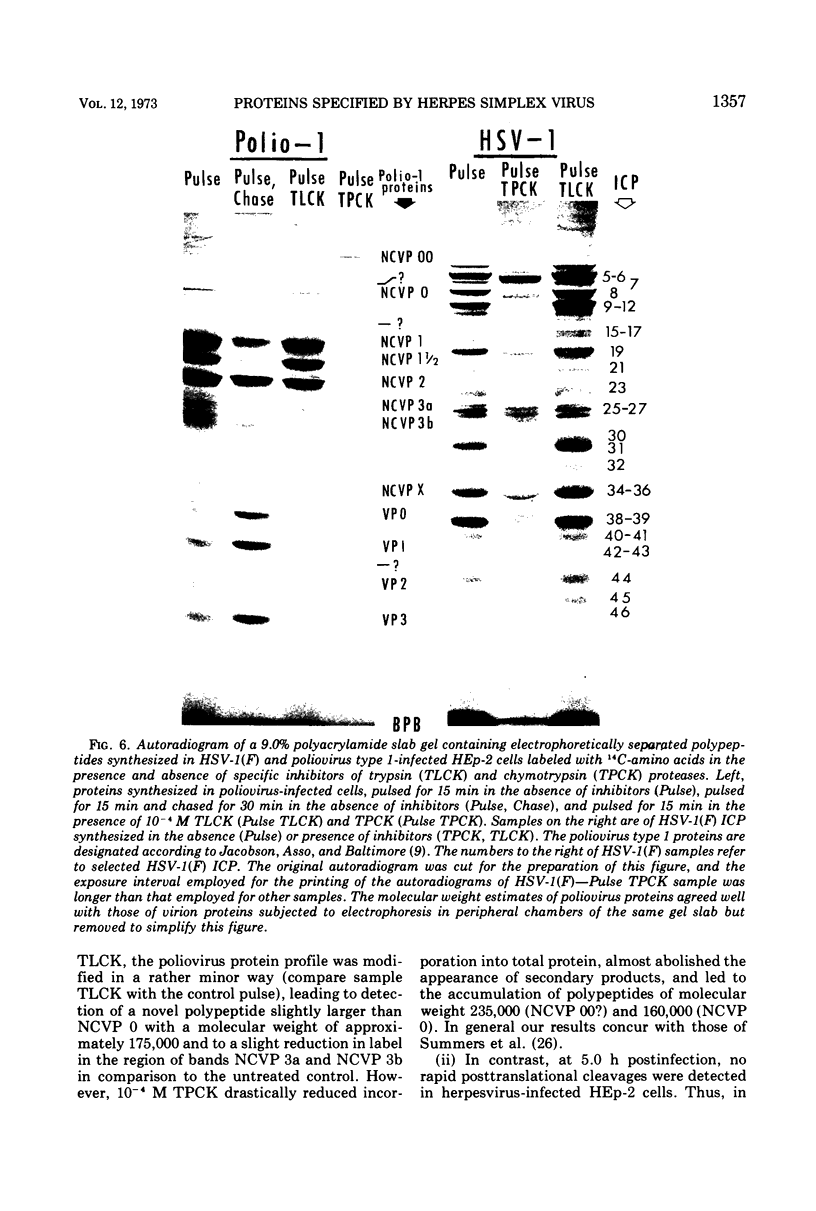
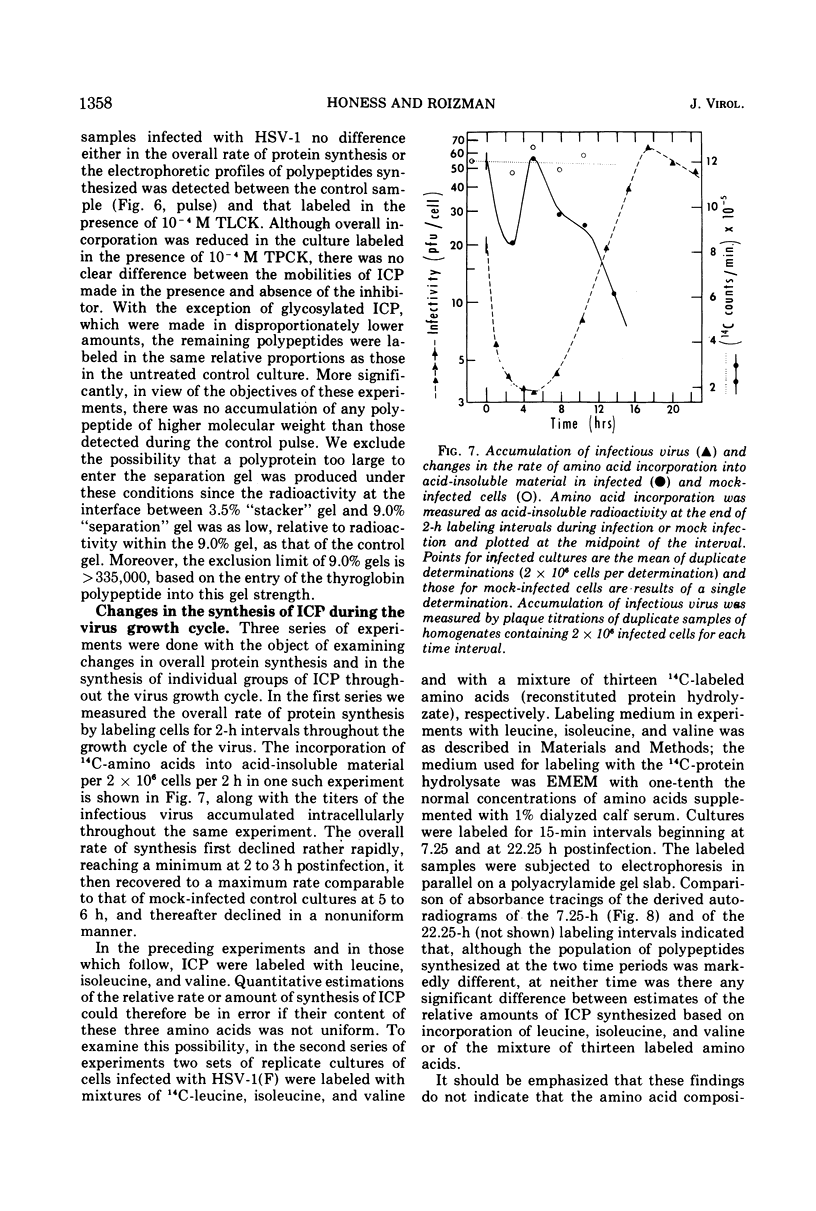
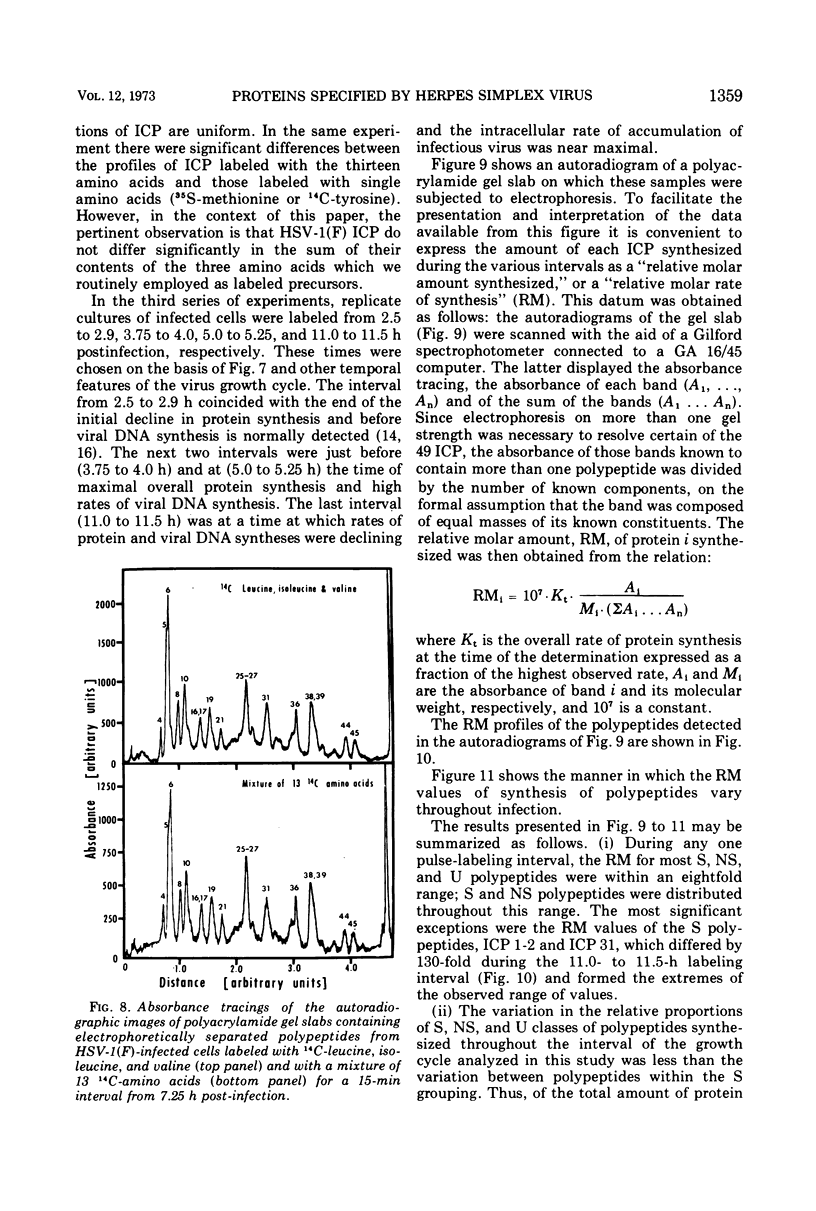
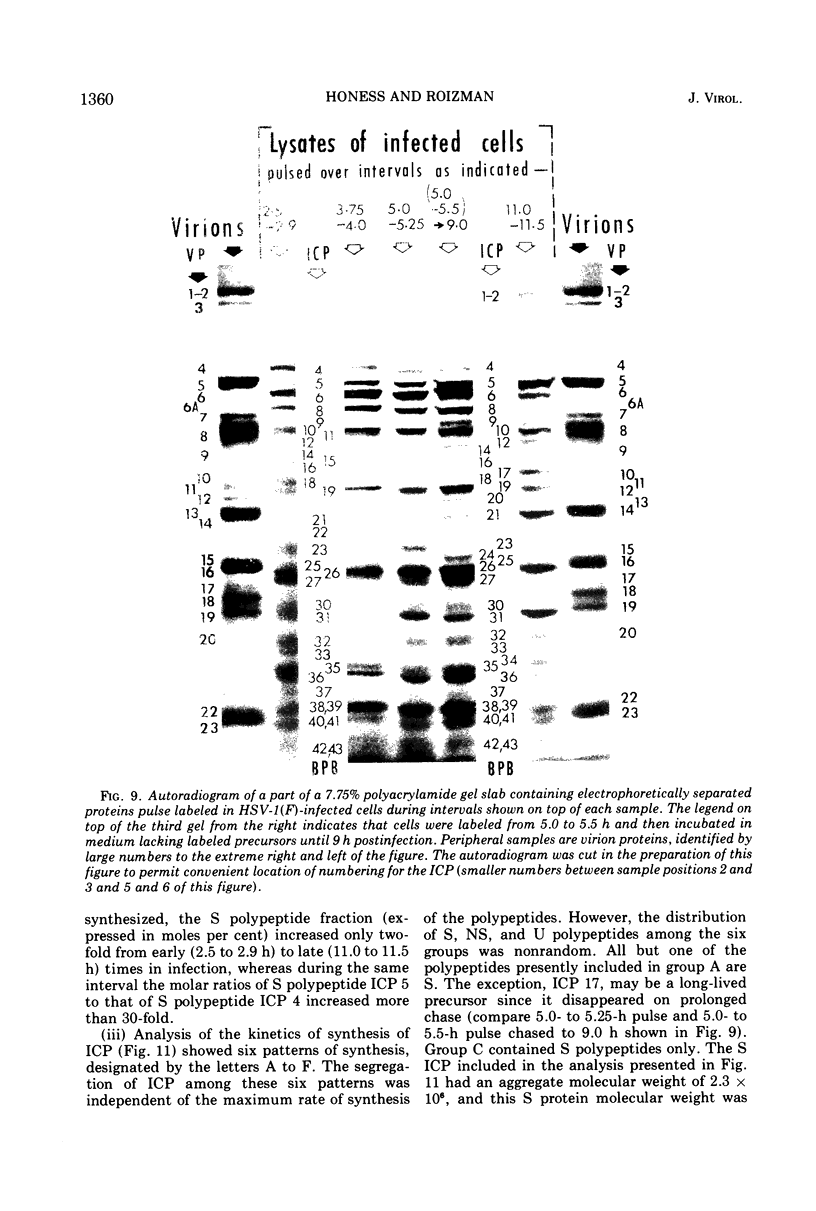
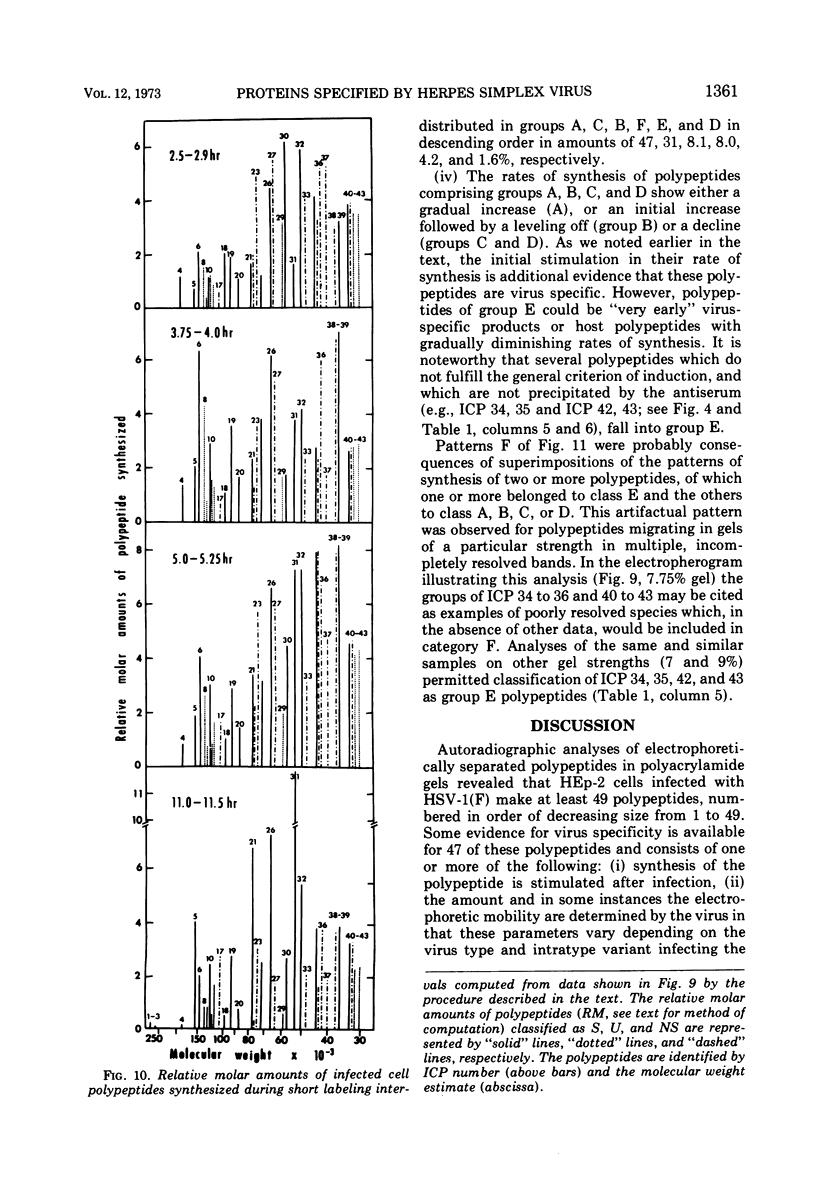
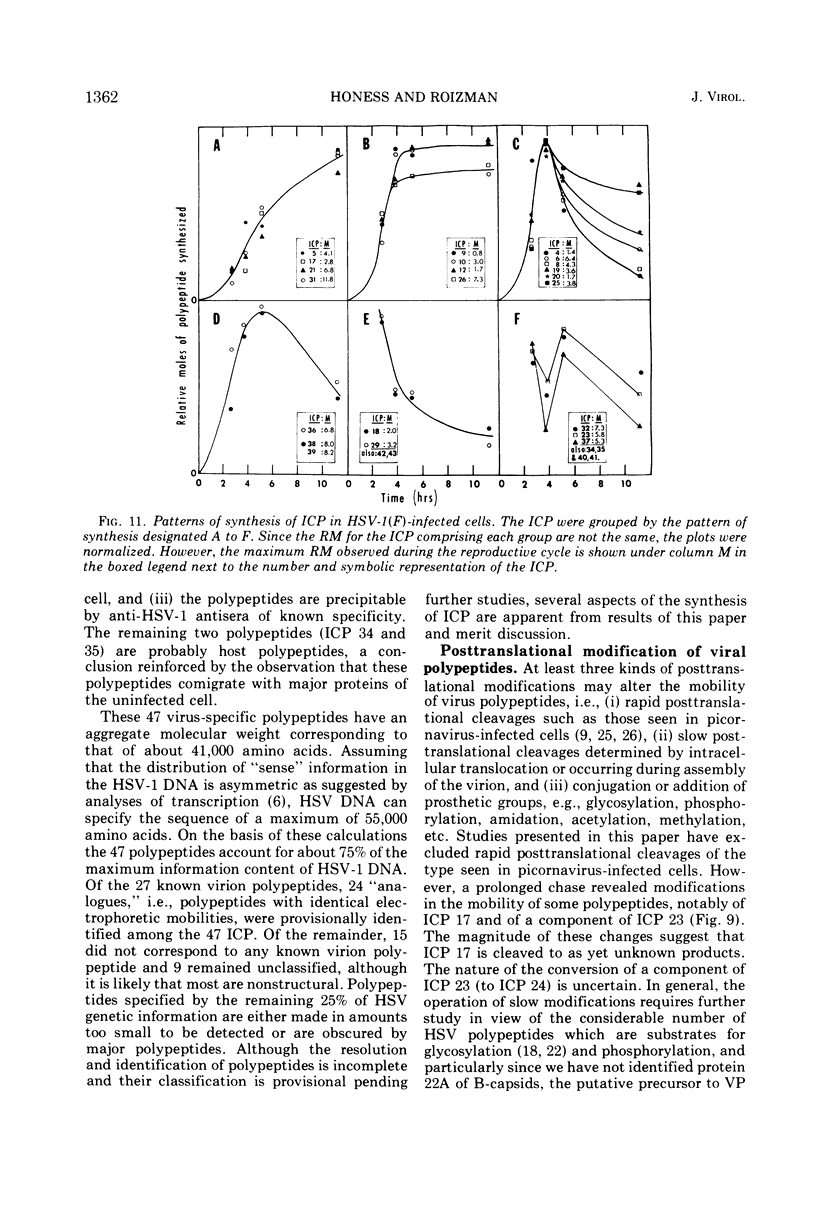
Analyses of polypeptides made in HEp-2 cells infected with herpes simplex virus type 1 by high-resolution polyacrylamide gel electrophoresis revealed the synthesis of at least 49 infected cell polypeptides (ICP) ranging in molecular weight from 15,000 to 280,000. Evidence for virus specificity based on increased rates of synthesis postinfection, immunological specificity, and viral control of mobility and rate of synthesis was available for 47 of the ICP. These 47 polypeptides can account for 75% of the virus genetic information assuming a DNA molecular weight of 108 and asymmetric transcription. On the basis of their mobility relative to virion proteins, the ICP were classified as structural (S, 23 polypeptides), nonstructural (NS, 16 polypeptides), and unassigned (U, 10 polypeptides). Analysis of the synthesis of the ICP revealed the following. (i) Rapid posttranslational cleavages of HSV proteins were not detected; in parallel experiments rapid posttranslational cleavages were readily demonstrated in poliovirus-infected cells and these were blocked by protease inhibitors. (ii) Slow posttranslational changes in the mobility of at least two polypeptides were observed. (iii) Analysis of the rates of synthesis of ICP examined at four intervals postinfection revealed regulation of the pattern and amount of ICP synthesized. ICP formed six classes (A to F) differing in their kinetics of synthesis. S and NS ICP were distributed nonrandomly among these classes. Thus, of the sum of S protein amino acid sequences apportioned among these kinetic classes, 47%, constituting class A and comprising “late” structural proteins, were characterized by progressively increasing rates of synthesis until at least 12 h postinfection; whereas “early” structural proteins constituting class C, amounting to 31% of the total amino acid sequences, were synthesized with initially increasing rates until 4 h postinfection and with declining rates thereafter. NS polypeptides and remaining S polypeptides were distributed among the other kinetic classes—B, D, E, and F. Control of protein abundance was evident in that the polypeptides were not made in equimolar amounts. However, S and NS polypeptides could not be differentiated on the basis of their molar rates of synthesis. The bulk of the detected polypeptides did not differ by more than eightfold in their molar rates of synthesis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Courtney R. J., McCombs R. M., Benyesh-Melnick M. Antigens specified by herpesviruses. II. Effect of arginine deprivation on the synthesis of cytoplasmic and nuclear proteins. Virology. 1971 Feb;43(2):356–365. doi: 10.1016/0042-6822(71)90308-4. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus: controls of transcription and of RNA abundance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2654–2658. doi: 10.1073/pnas.69.9.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Silverstein S., Cassai E., Roizman B. RNA synthesis in cells infected with herpes simplex virus. VII. Control of transcription and of transcript abundancies of unique and common sequences of herpes simplex virus 1 and 2. J Virol. 1973 Jun;11(6):886–892. doi: 10.1128/jvi.11.6.886-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Kaplan A. S., Shimono H., Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus. 3. Relative amino acid content of various proteins formed after infection. Virology. 1970 Jan;40(1):90–101. doi: 10.1016/0042-6822(70)90382-x. [DOI] [PubMed] [Google Scholar]
- Korant B. D. Cleavage of viral precursor proteins in vivo and in vitro. J Virol. 1972 Oct;10(4):751–759. doi: 10.1128/jvi.10.4.751-759.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr A physical difference between two strains of herpes simplex virus apparent on sedimentation in cesium chloride. Virology. 1961 Sep;15:75–79. doi: 10.1016/0042-6822(61)90079-4. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr THE MULTIPLICATION OF HERPES SIMPLEX VIRUS. II. THE RELATION BETWEEN PROTEIN SYNTHESIS AND THE DUPLICATION OF VIRAL DNA IN INFECTED HEP-2 CELLS. Virology. 1964 Feb;22:262–269. doi: 10.1016/0042-6822(64)90011-x. [DOI] [PubMed] [Google Scholar]
- Roizman B., Spear P. G. Preparation of herpes simplex virus of high titer. J Virol. 1968 Jan;2(1):83–84. doi: 10.1128/jvi.2.1.83-84.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage T., Roizman B., Heine J. W. Immunological specificity of the glycoproteins of herpes simplex virus subtypes 1 and 2. J Gen Virol. 1972 Oct;17(1):31–48. doi: 10.1099/0022-1317-17-1-31. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Silverstein S., Bachenheimer S. L., Frenkel N., Roizman B. Relationship between post-transcriptional adenylation of herpes virus RNA and messenger RNA abundance. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2101–2104. doi: 10.1073/pnas.70.7.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Kellejmroian B. Proteins spcified by herpes simplex virus. II. Viral glycoprotins associated with cellular membranes. J Virol. 1970 Feb;5(2):123–131. doi: 10.1128/jvi.5.2.123-131.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. The proteins specified by herpes simplex virus. I. Time of synthesis, transfer into nuclei, and properties of proteins made in productively infected cells. Virology. 1968 Dec;36(4):545–555. doi: 10.1016/0042-6822(68)90186-4. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Shaw E. N., Stewart M. L., Maizel J. V., Jr Inhibition of cleavage of large poliovirus-specific precursor proteins in infected HeLa cells by inhibitors of proteolytic enzymes. J Virol. 1972 Oct;10(4):880–884. doi: 10.1128/jvi.10.4.880-884.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. H., Shedden W. I., Elliot A., Tetsuka T., Wildy P., Bourgaux-Ramoisy D., Gold E. Virus specific antigens in mammalian cells infected with herpes simplex virus. Immunology. 1966 Oct;11(4):399–408. [PMC free article] [PubMed] [Google Scholar]