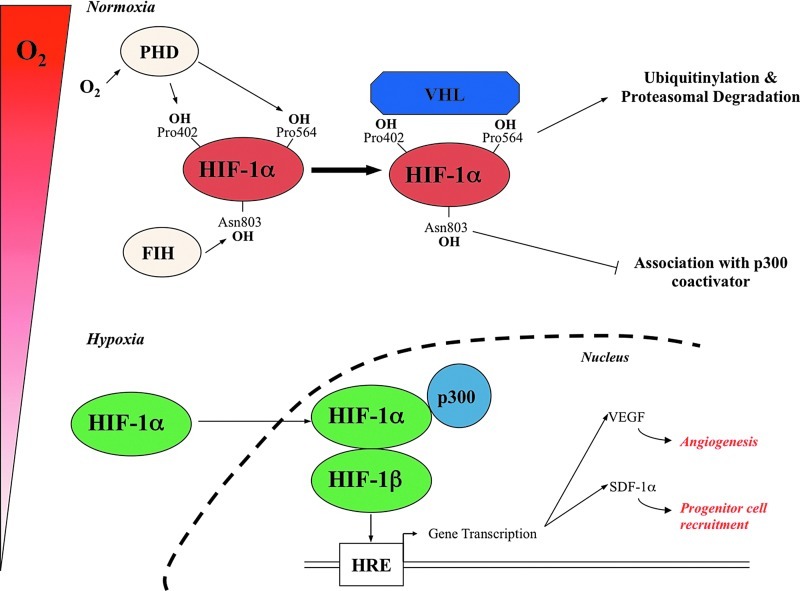
FIG. 2.
Oxygen-dependent post-translational modifications of hypoxia inducible factor (HIF)-1α levels. Under normoxic conditions, HIF-1α is hydroxylated (OH) on proline residues 402 and 564 (Pro) in the oxygen-dependent degradation domain by specific Prolyl Hydroxylase Domain proteins (PHDs). Once hydroxylated, HIF-1α is recognised by the von Hippel-Lindau protein (VHL), which is part of an ubiquitin–ligase complex known as E3 ligase complex, which targets HIF-1α for polyubiquitination and subsequent proteasomal degradation. The asparagine 803 (Asn 803) residue of HIF1α is also hydroxylated by FIH, which impairs the interaction with the transcriptional coactivator p300/CREB binding protein (p300) with the HIF-1α C-terminal transactivation domain. When oxygen becomes limited, the proline residues are no longer hydroxylated and HIF-1αɛσχαπɛσ δɛγραδατιoν, is translocated into the nucleus, where it dimerises with HIF-1β ανδ binds to hypoxia-responsive elements (HRE) to the promoter or enhancer sequences of target genes. In addition, interaction with p300/CREB binding protein, due to inhibition of Asn 803 hydroxylation, increases the transcriptional activity of HIF. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)