Abstract
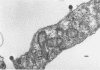
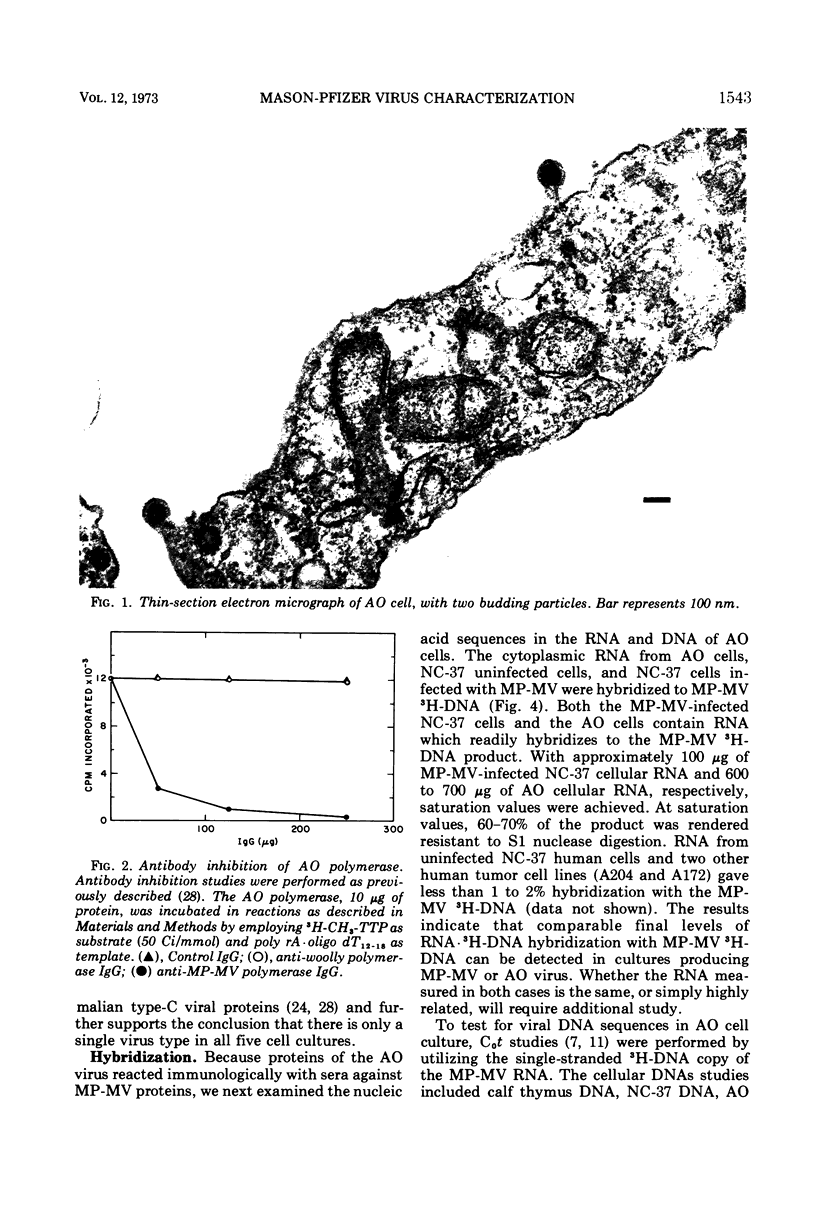
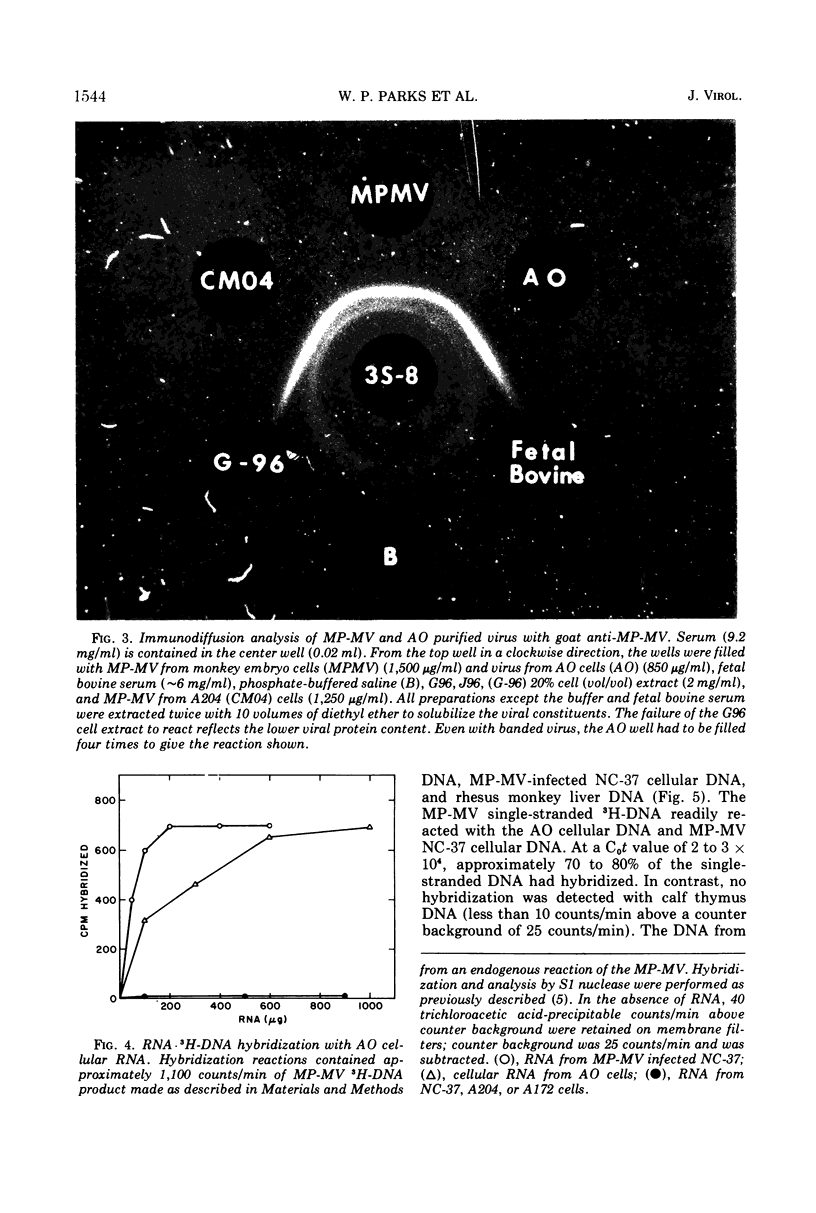
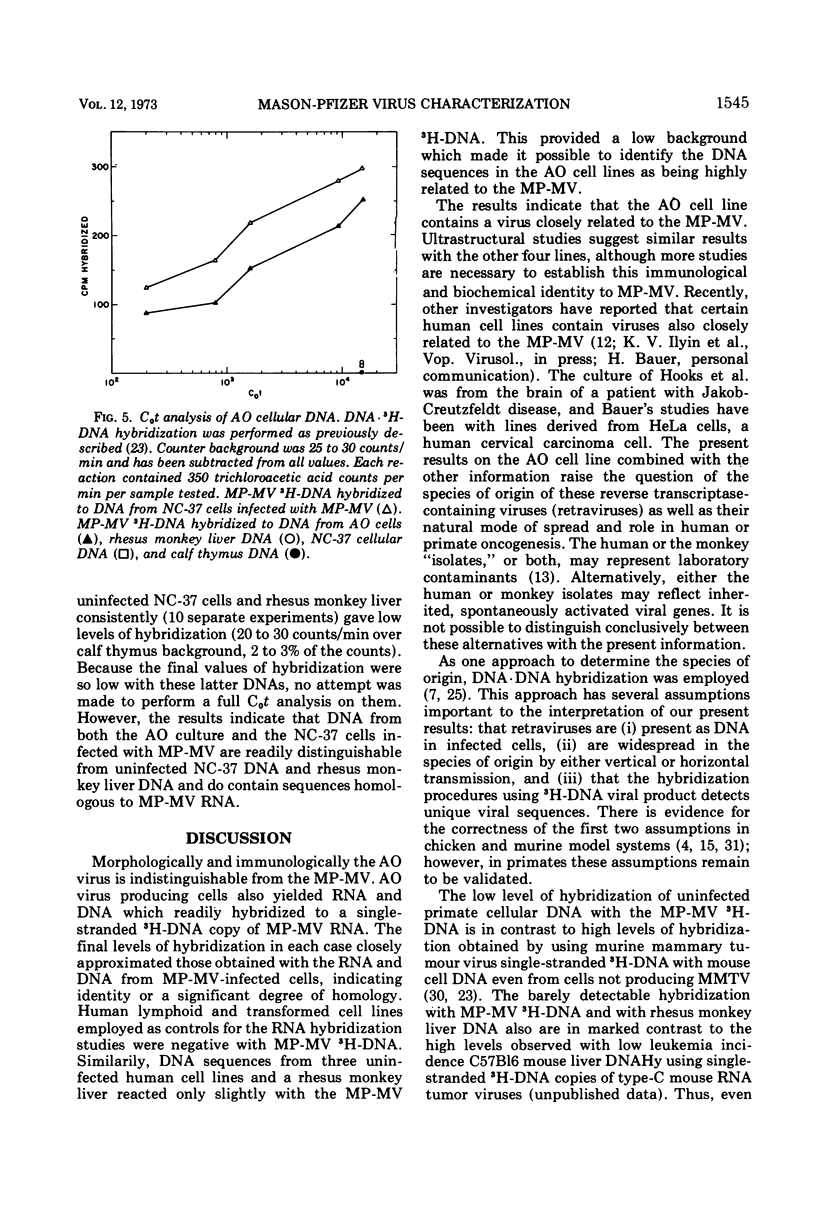
Mason-Pfizer monkey virus (MP-MV) is a RNA virus with an RNA-instructed DNA polymerase first isolated from a rhesus monkey mammary adenocarcinoma in 1970. Until recently, there have been no other isolates. A continuous human amnion cell line, AO, was found to be producing a virus indistinguishable or closely related to the Mason-Pfizer virus as measured by morphological, immunological, and biochemical methods. By thin-section electron microscopy, the extracellular virus particle in AO line is 115 to 130 nm in diameter and has a preformed nucleoid (80 to 90 nm) before budding, properties which are also characteristic of MP-MV. Two proteins of the virus from the AO line were studied. By immunodiffusion, sera which react specifically with MP-MV give a line of identity with virus from the AO line. The AO viral RNA-instructed DNA polymerase purified by phosphocellulose chromatography was specifically inhibited by anti-MP-MV polymerase sera, and the AO cells contained both DNA and RNA sequences related to MP-MV 3H-DNA. Viruses thus far indistinguishable from MP-MV have also recently been found by others in different human lines, raising again the question of the species of origin of MP-MV. Because the virus in the AO cells cannot be differentiated from MP-MV, we attempted to determine the origin of MP-MV virus by measuring DNA sequences related to MP-MV 3H-DNA in uninfected human and rhesus monkey cells. The quantity of MP-MV-like DNA sequences in uninfected primate tissues was found to be much lower than the amount of DNA sequences of murine type-B or type-C viruses in uninfected murine tissues. Thus, it was not possible to determine whether the virus produced by AO cells or MP-MV was of human or monkey origin, or both.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andzhaparidze O. G., Liozner A. L., Stepanova L. G., Shukhmina N. R., Shevelev B. I. Obnaruzhenie antigennogo kompleksa onkogennogo RNK-soderzhashchego virusa (shtamm LPV) u bol'nykh leikozom. Vopr Virusol. 1972 Nov-Dec;17(6):686–690. [PubMed] [Google Scholar]
- Andzhaparidze O. G., Lotte V. D., Iurovskaia G. B. Ul'trastruktura diploidnykh kletok cheloveka, transformirovannykh krov'iu bol'nykh gemotsitoblastozom. Vopr Virusol. 1970 Sep-Oct;15(5):584–589. [PubMed] [Google Scholar]
- Aoki T., Herberman R. B., Johnson P. A., Liu M., Sturm M. M. Wild-type gross leukemia virus: classification of soluble antigens (GSA). J Virol. 1972 Dec;10(6):1208–1219. doi: 10.1128/jvi.10.6.1208-1219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNHARD W. Electron microscopy of tumor cells and tumor viruses; a review. Cancer Res. 1958 Jun;18(5):491–509. [PubMed] [Google Scholar]
- Baluda M. A., Drohan W. N. Distribution of deoxyribonucleic acid complementary to the ribonucleic acid of avian myeloblastosis virus in tissues of normal and tumor-bearing chickens. J Virol. 1972 Nov;10(5):1002–1009. doi: 10.1128/jvi.10.5.1002-1009.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Bykovskii A. F., Miller G. G., Klitsunova N. V., Gorokhova L. V., Zakhaleva V. A. Morfologiia onkonornavirusov tipov A, B, i C perevivaemykh linii kletok cheloveka i zhivotnykh. Vopr Virusol. 1973 Mar-Apr;18(2):215–221. [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- Dalton A. J., Stewart S. E. Intracisternal A particles and C particles. Science. 1972 Apr 21;176(4032):319–319. doi: 10.1126/science.176.4032.319. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Hooks J., Gibbs C. J., Jr, Chopra H., Lewis M., Gajdusek D. C. Spontaneous tranformation of human brain cells grown in vitro and description of associated viurs particles. Science. 1972 Jun 30;176(4042):1420–1422. doi: 10.1126/science.176.4042.1420. [DOI] [PubMed] [Google Scholar]
- Howk R. S., Rye L. A., Killeen L. A., Scolnick E. M., Parks W. P. Characterization and separation of viral DNA polymerase in mouse milk. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2117–2121. doi: 10.1073/pnas.70.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Il'in K. V., Bykovskii A. F., Zhdanov V. M. Onkornavirus tipa B iz kletok kartsinomy gortani cheloveka. Vopr Virusol. 1972 Jul-Aug;17(4):494–499. [PubMed] [Google Scholar]
- Ilyin K. V., Bykovsky A. F., Zhdanov V. M. An oncornavirus isolated from human cancer cell line. Cancer. 1973 Jul;32(1):89–96. doi: 10.1002/1097-0142(197307)32:1<89::aid-cncr2820320112>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER H. M., Jr, BROOKS B. E., DOUGLAS R. D., ROGERS N. G. Ecology of measles in monkeys. Am J Dis Child. 1962 Mar;103:307–313. doi: 10.1001/archpedi.1962.02080020319025. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Murine mammary tumor cell clones with varying degrees of virus expression. Virology. 1973 Sep;55(1):163–173. doi: 10.1016/s0042-6822(73)81018-9. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Noon M. C., Watson C. J., Kawakami T. G. Radioimmunoassay of mammalian type-C polypeptides. IV. Characterization of woolly monkey and gibbon viral antigens. Int J Cancer. 1973 Jul 15;12(1):129–137. doi: 10.1002/ijc.2910120114. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Radioimmunoassay of mammalian type-C viral proteins: interspecies antigenic reactivities of the major internal polypeptide. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1766–1770. doi: 10.1073/pnas.69.7.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht R. M., Goodman N. C., Spiegelman S. Determination of natural host taxonomy of RNA tumor viruses by molecular hybridization: application to RD-114, a candidate human virus. Proc Natl Acad Sci U S A. 1973 May;70(5):1437–1441. doi: 10.1073/pnas.70.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science. 1971 Nov 19;174(4011):840–843. doi: 10.1126/science.174.4011.840. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Todaro G. J., Aaronson S. A. Immunological characterization of primate C-type virus reverse transcriptases. Nat New Biol. 1972 Jan 12;235(54):35–40. doi: 10.1038/newbio235035a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Todaro G. J. Reverse transcriptases of primate viruses as immunological markers. Science. 1972 Sep 22;177(4054):1119–1121. doi: 10.1126/science.177.4054.1119. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Nowinski R. C., Sarker N. H. Mammary tumour virus specific nucleotide sequences in mouse DNA. Nat New Biol. 1972 Aug 9;238(84):189–191. doi: 10.1038/newbio238189a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Philipson L., Darnell J. E. Processing of adenovirus specific nuclear RNA during virus replication. Virology. 1972 Oct;50(1):27–34. doi: 10.1016/0042-6822(72)90342-x. [DOI] [PubMed] [Google Scholar]
- Zhdanov V. M., Soloviev V. D., Bektemirov T. A., Filatov F. P., Bykovsky A. F. Isolation of a leukovirus from a continuous human cell line. Arch Gesamte Virusforsch. 1972;39(4):309–316. doi: 10.1007/BF01241009. [DOI] [PubMed] [Google Scholar]
- Zhdanov V. M., Soloviev V. D., Bektemirov T. A., Ilyin K. V., Bykovsky A. F., Mazurenko N. P., Irlin I. S., Yershov F. I. Isolation of oncornaviruses from continuous human cell cultures. Intervirology. 1973;1(1):19–26. doi: 10.1159/000148828. [DOI] [PubMed] [Google Scholar]