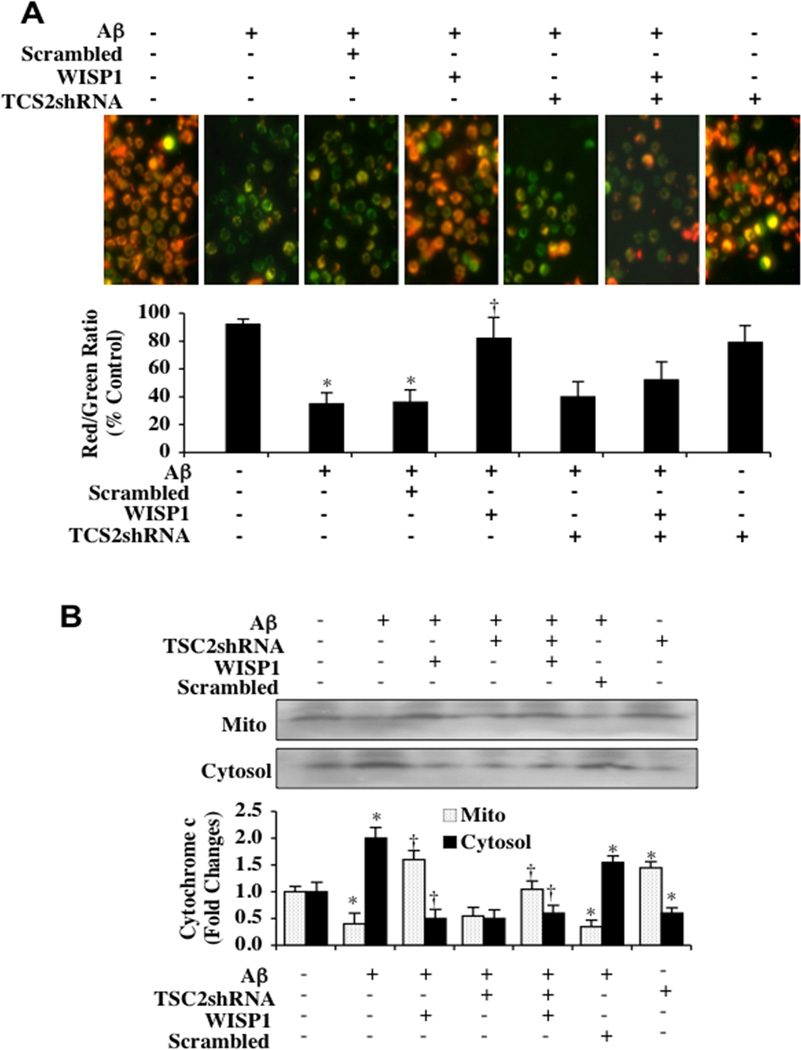
Fig. (5). TSC2 is necessary for WISP1 to prevent mitochondrial depolarization and cytochrome c release during Aβ exposure.
(A) Aβ (10 µM) exposure produced a significant decrease in the red/green fluorescence intensity ratio of mitochondria within 6 hours using the cationic membrane potential indicator JC-1 when compared with untreated control cultures, demonstrating that Aβ results in mitochondrial membrane depolarization. WISP (10 ng/ml) administrated 1 hour prior to Aβ significantly increased the red/green fluorescence intensity of mitochondria in microglia, illustrating that mitochondrial membrane potential was restored. Loss of TSC2 with transfection of TSC2 shRNA significantly reduced the ability of WISP1 to prevent mitochondrial depolarization during Aβ exposure. Gene silencing of TSC2 did not alter mitochondrial membrane depolarization in the absence of Aβ exposure. The relative ratio of red/green fluorescent intensity of mitochondrial staining was measured in 3 independent experiments with analysis performed using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image) (*P<0.01 vs. untreated control; †P <0.01 vs. Aβ). (B) Equal amounts of mitochondrial (Mito) or cytosol protein extracts (50 µg/lane) were immunoblotted demonstrating that WISP1 administration significantly prevented cytochrome c release from mitochondria during Aβ exposure (*P<0.01 vs. untreated control; †P <0.01 vs. Aβ). Transfection with TSC2 shRNA attenuated the ability of WISP1 to prevent mitochondrial release of cytochrome c. Gene silencing of TSC2 did not alter cytochrome c release in the absence of Aβ exposure. Non-specific scrambled shRNA did not affect mitochondrial depolarization or cytochrome c release during Aβ exposure when compared to Aβ exposure alone.. Each data point represents the mean and SD from 3 experiments.