Abstract
Recent in vitro experiments demonstrated that arteries under increased internal pressure or decreased axial stretch may buckle into the tortuous pattern that is commonly observed in aging or diseased arteries in vivo. It suggests that buckling is a possible mechanism for the development of artery tortuosity. Vascular tone has significant effects on arterial mechanical properties but its effect on artery buckling is unknown. The objective of this study was to determine the effects of smooth muscle cell contraction on the critical buckling pressure of arteries. Porcine common carotid arteries were perfused in an ex vivo organ culture system overnight under physiological flow and pressure. The perfusion pressure was adjusted to determine the critical buckling pressure of these arteries at in vivo and reduced axial stretch ratios (1.5 and 1.3) at baseline and after smooth muscle contraction and relaxation stimulated by norepinephrine and sodium nitroprusside, respectively. Our results demonstrated that the critical buckling pressure was significantly higher when the smooth muscle was contracted compared with relaxed condition (97.3mmHg versus 72.9mmHg at axial stretch ratio of 1.3 and 93.7mmHg vs 58.6mmHg at 1.5, p<0.05). These results indicate that arterial smooth muscle cell contraction increased artery stability.
Keywords: critical buckling pressure, mechanical instability, smooth muscle cell contraction, vascular tone, stability
Introduction
Tortuous arteries are seen in various locations of the vasculature, such as the internal carotid arteries, iliac arteries, coronary arteries, retinal arteries, and conjunctival arteries (Metz et al. 1961; Jakob et al. 1996; Del Corso et al. 1998; Amemiya and Bhutto 2001; Cheung et al. 2001; Owen et al. 2008; Han 2012). Arterial tortuosity has been associated with high blood pressure, aging, atherosclerosis, and other pathological changes (Del Corso et al. 1998; Pancera et al. 2000; Han 2012). Studies have also demonstrated that axial elongation and reduced axial tension could lead to artery tortuosity (Leipzig and Dohrmann 1986; Jackson et al. 2005), suggesting a possible role of mechanical stress in the development of artery tortuosity. Recent studies, on the other hand, have demonstrated that arteries buckle under increased lumen pressure, weakened wall and decreased axial tension (Han 2007; Han 2008; Han 2011; Liu and Han 2012). Model analysis and in vitro experiments demonstrated that buckled arteries exhibit a tortuous pattern suggesting buckling as a possible mechanism for the development of artery tortuosity (Han 2007; Han 2009; Han 2012; Lee et al. 2012). Therefore, it is important to understand the behavior of artery buckling.
Computational and experimental studies have demonstrated that the critical buckling pressure, at which the arteries start to deform into wavy shapes, depends on artery dimensions, wall stiffness and axial stretch ratio. Arteries with larger diameters, thicker walls, and higher levels of axial tension all exhibit more stability (Han 2007; Martinez et al. 2010; Lee et al. 2012). In vivo, mechanical properties of the artery wall are also influenced by smooth muscle tone. Vasomotion driven by smooth muscle cells (SMC) contraction and relaxations is one mechanism through which the arteries regulate local blood pressure and flow (Humphrey 2002). Smooth muscle contraction decreases lumen diameter (vasoconstriction) but increases artery stiffness (Wilkinson and McEniery 2004; Tremblay et al. 2010). However, it is unclear how SMC tone affects artery stability.
Accordingly, the objective of this study was to determine how SMC tone affects the critical buckling of the artery, by examining the critical buckling pressures of arteries challenged with norepinephrine (NE) and sodium nitroprusside (SNP).
Materials and Methods
Porcine common carotid arteries were collected from farm pigs post-mortem (6–7 months, 100–150 kg) with the approval by the Texas Department of State Health Service and the University of Texas at San Antonio (UTSA) Institutional Biosafety Committee. Arteries were transported to the lab in ice cold phosphate buffered saline (PBS) supplemented with 1.4% antibiotic/antimycotic (Gibco, Grand Island, NY). Excess loose connective tissue was removed and an inflation test was performed to verify arterial wall integrity. The arteries were tied onto cannulas in a tissue chamber of an ex vivo organ culture system (Lee et al. 2010a; Lee et al. 2010b) and perfused with DMEM (Sigma, St. Louis, MO) supplemented with sodium bicarbonate (3.7g/L), antibiotic/antimycotic (1.4%, Gibco), L-glutamine (1%, Sigma), calf serum(10%, Sigma), HEPES buffer(2.5%, Gibco) and Dextran (50–55g/L, Sigma) to increase viscosity to a physiological level (~4cP). The arteries were immersed in the same media without Dextran and cultured overnight (~12 hrs, 37°C, and 5% CO2) under a flow rate of 160 ml/min and a pressure of 80±2 mmHg to allow the arteries to regain their basal tone. Our previous studies have demonstrated that vasomotor responses were similar under steady or pulsatile pressure after 3 days in organ culture (Hayman et al. 2012).
Two groups of arteries were cultured overnight at a physiological axial stretch ratio of 1.5 (n=5) (which was at their in vivo lengths (Han et al. 2003)) and a sub-physiological axial stretch ratio of 1.3 (n=7). Buckling tests, as described previously (Martinez et al. 2010; Lee et al. 2012), were performed at the stretch ratios under which the arteries were cultured. Briefly, perfusion pressure in the arteries was gradually reduced to a low level (~15 mmHg) by fully loosening the resistant clamp. The pressure was then gradually increased until buckling occurred and a visually significant deflection (3–8 mm) occurred post-buckling. The buckling test was performed for arteries at baseline, and then repeated after the arteries were contracted by NE (10−5 M for ~5 min). It was repeated again after the arteries were relaxed by SNP (10−5 M for ~5 min) (Hayman 2011). Our previous studies have demonstrated that arteries were fully contracted or relaxed at these concentrations (Lee et al. 2010a; Hayman et al. 2012). The buckling process was recorded using a Sony DCR-SX63 camcorder. Arterial outer diameter and deflection (displacement of the central axis at the middle of the arterial segments) were measured from images extracted from the video at increments of ~ 10 mmHg using Image-Pro Plus.
Artery diameters were measured over the range of pressures in the buckling test (70 to 170 mmHg and 20–120 mmHg for the groups of stretch ratios 1.3 and 1.5, respectively). Both the diameter at a lumen pressure of 80 mmHg and the average over the entire range were used to describe the contraction and relaxation of the arteries. Artery contraction was calculated as the difference between the baseline and the NE-induced contracted diameters divided by the baseline diameter. Artery relaxation was quantified as the difference between the NE-induced contracted diameter and the SNP-induced relaxaed diameter divided by the NE-induced contracted diameter. The basal tone was taken as the percent diameter increase from the baseline to the SNP-induced relaxaed condition. The critical buckling pressure was taken as the pressure at which the deflection reached 0.5 mm; this point was interpolated from the pressure deflection curve using the TREND function in EXCEL.
The average critical buckling pressure for each group was calculated and statistical significance was determined using a two-way ANOVA followed by a Tukey’s post hoc analysis (JMP version 8).
Results
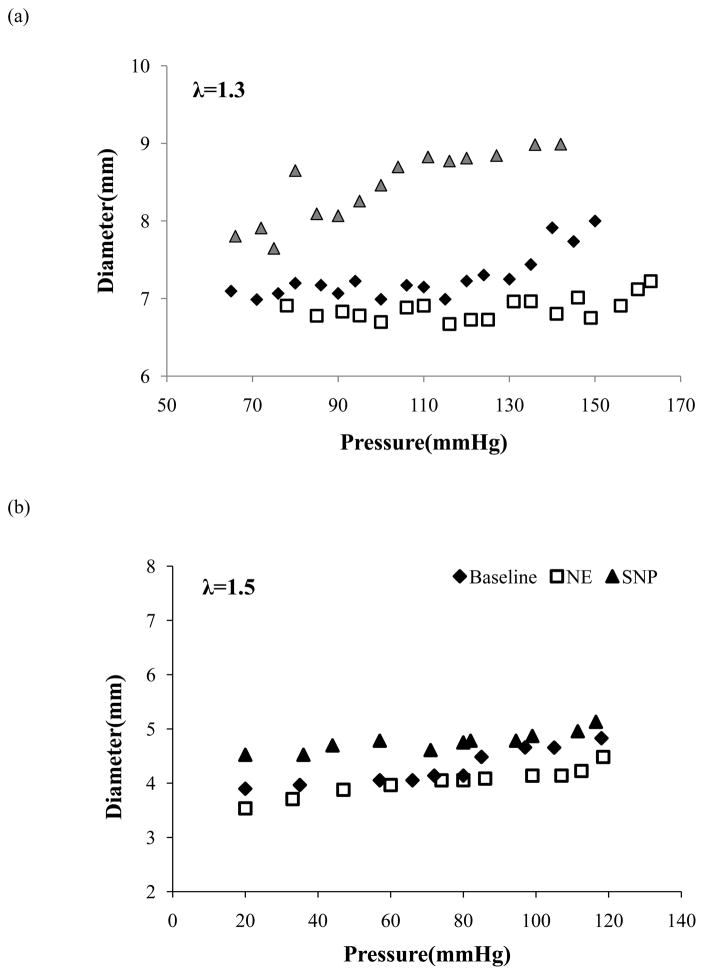
Arteries regained their vasomotor tone after being cultured overnight. When pressurized, arterial diameter increased with increasing pressure, at all axial stretch ratios and agonists. Compared to baseline, arterial diameter was decreased across all arteries by NE-induced SMC contraction and increased by SNP-induced SMC relaxation (Figure 1). For the artery group tested under axial stretch ratio 1.3, the contraction and relaxation (mean ± SD, n=6) averaged over the entire pressure range (70–170 mmHg) were 6.0 ± 8.4 % and 13.9 ± 9.0%, in response to NE and SNP, respectively (p<0.05), with a basal tone of 10.4 ± 9.3 %. At a lumen pressure of 80 mmHg, the contraction and relaxation (mean ± SD, n=6) were 4.9 ± 1.3% and 5.7 ± 1.2%, in response to NE and SNP, with a basal tone of 5.1 ± 0.9%. Similarly, for the artery group cultured under axial stretch ratio 1.5, the contraction and relaxation (mean ± SD, n=5) averaged over the entire pressure range (20 – 120 mmHg) were 3.7% ± 1% and 22.7% ± 7%, in response to NE and SNP, respectively (p<0.05), with a basal tone of 18% ± 9%. At a lumen pressure of 80 mmHg, the contraction and relaxation (mean ± SD, n=5) were 5.9 ± 1.1% and 8.1 ± 1.9% in response to NE and SNP, respectively, with a basal tone of 5.9 ± 1.7%.
Figure 1.
Comparison of outer diameter change as a function of lumen pressure of representative arteries at baseline, after administration of norepinephrine (NE), and sodium nitroprusside (SNP). The arteries were tested at a stretch ratio of 1.3 (a) and 1.5 (b), respectively.
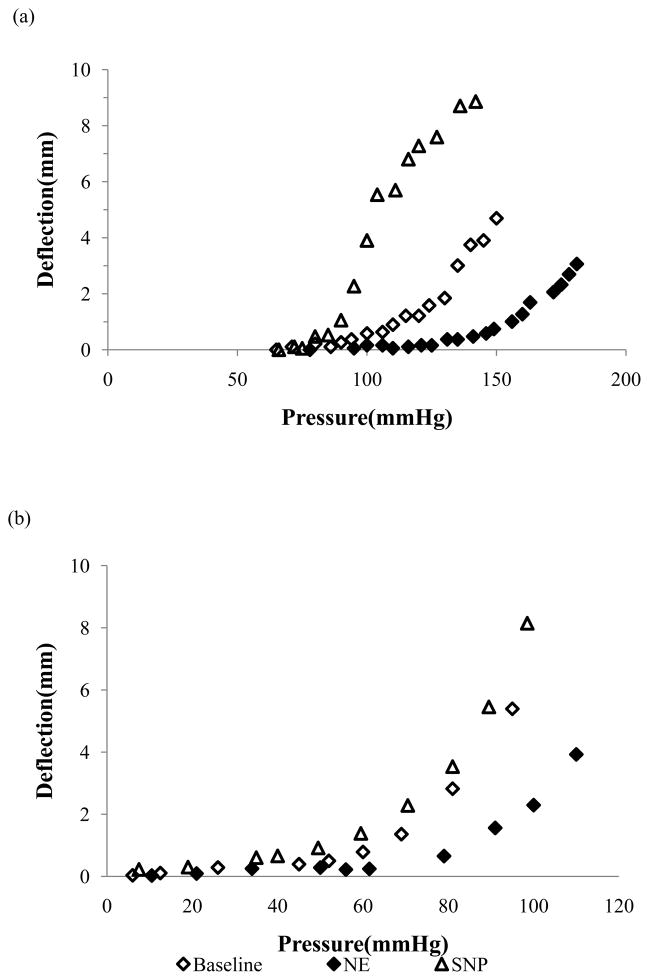
During the buckling test, artery deflection was initiated at a critical pressure and gradually increased with increasing lumen pressure (Figure 2). NE-induced contraction shifted the pressure-deflection curve to the right while SNP-induced relaxation shifted the curve to the left, indicating that NE-induced SMC contraction increased the critical pressure and SNP-induced SMC relaxation decreased the critical buckling pressure (Figure 3). The pressure-deflection curves all exhibit a nonlinear accelerated increase immediately at post-buckling but tend to slow down at higher pressures further beyond buckling (Figure 3a). This trend has been observed previously (Lee 2011; Lee et al. 2012), so we did not measure the high pressure range for most of these arteries. In comparison, the critical buckling pressures of the arteries at NE-induced contraction was significantly higher than those at SNP-induced relaxation condition, at both axial stretch ratios of 1.3 and 1.5 (p<0.05, Figure 4). No significant difference was observed between the critical buckling pressures of arteries at the baseline and SNP-induced relaxation condition.
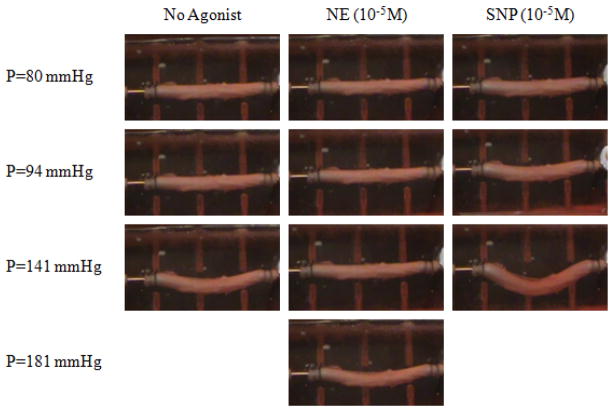
Figure 2.
Photographic illustration of the buckling process of an artery at baseline, after administration of norepinephrine (NE), and after sodium nitroprusside (SNP). The axial stretch ratios were 1.3.
Figure 3.
Comparison of arterial deflection as functions of lumen pressure of representative arteries at baseline, after administration of norepinephrine (NE) and sodium nitroprusside (SNP). The arteries were tested at a stretch ratio of 1.3 (a) and 1.5 (b), respectively.
Figure 4.
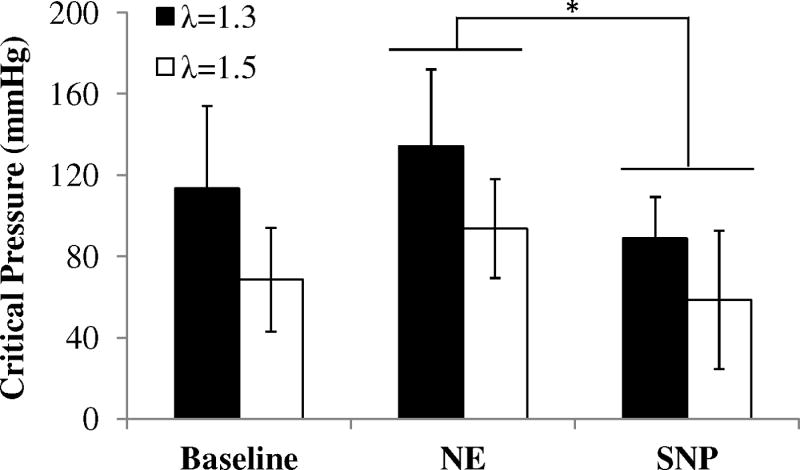
Comparison of critical buckling pressure (mean ± SD) measured for arteries under axial stretch ration of 1.3 (n=7) and 1.5 (n=5) at baseline, after administration of norepinephrine (NE) and sodium nitroprusside (SNP), * p < 0.05.
Discussion
Our results indicate that contraction of the arteries significantly increases the critical buckling pressure compared with the dilated state. Interestingly, the critical buckling pressure of arteries at baseline showed no significant difference from that at the fully relaxed condition. This suggests that the critical buckling pressure measured in vitro in fully relaxed arteries is close to in vivo value though it may slightly underestimate the critical buckling pressure in vivo.
Previous studies have shown that vascular smooth muscle cells, as the main component of the vessel wall, play a key role in vascular development, differentiation, vessel wall maintenance and remodeling, as well as pathological processes such as atherosclerosis, restenosis and aneurysm (Majesky et al. 2011; Torella et al. 2011). It is well known that SMC contraction affects arterial wall mechanical stiffness and the lumen diameter. In this study, we demonstrated that SMC contraction and relaxation also increase and decrease the critical buckling pressure, respectively. It is interesting to note that contracted arteries exhibited both a higher level of stability and a decrease in diameter. This could be due to the increases in wall thickness and stiffness associated with SMC contraction. These increases outweigh the decrease in diameter and led to a net effect of increased critical pressure.
The critical pressures in the second group with an axial stretch ratio of 1.5 were slightly lower than the first group with an axial stretch ratio of 1.3. Our previous studies have demonstrated that for the same artery, its critical pressure at a stretch ratio of 1.5 is higher than the critical pressure at a stretch ratio of 1.3 (Han 2007; Lee et al. 2012). In fact, additional tests of the first group did confirm that in its basal contraction state each artery has a higher critical pressure at a stretch ratio of 1.5 compared to 1.3. However, the contraction and relaxation responses were significantly weaker in arteries that had already been challenged with NE and SNP once which necessitated the use of separate groups of arteries for each stretch ratio. Thus, we used two separate groups of arteries to test the effects of vasomotor function at stretch ratios of 1.5 and 1.3. This design was used to ensure the strength of the contraction and relaxation responses; however, this made it difficult to compare the values at 1.3 and 1.5 directly. The lower critical buckling pressure in the 1.5 group may be due to the dimensional differences in the vessel segments used. Another limitation of the study was that the effects of surrounding tissue support as seen in vivo were ignored. Our previous results have showed that surrounding tissue support would increase vessel stability and change buckling pattern into a higher mode (Lee et al. 2012), but we expect the effect of SMC contraction on the critical pressure would be similar since the changes in diameter, wall thickness, and stiffness due to SMC contraction would be the same (not affected by the surrounding tissue support).
In conclusion, our experimental results suggest that SMC contraction increases artery stability, these results improve our understanding of the stability of arteries in vivo and the development of artery tortuosity.
Acknowledgments
This work was supported by CAREER award #0644646 from the National Science Foundation and grant HL095852 and HHSN 268201000036C (N01-HV-00244) for the San Antonio Cardiovascular Proteomics Center from the National Institutes of Health. We thank Wiatrek at Poth and Granzins at New Braunfels, TX for their kind help.
Footnotes
Conflict of interest
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amemiya T, I, Bhutto A. Retinal vascular changes and systemic diseases: corrosion cast demonstration. Ital J Anat Embryol. 2001;106(2 Suppl 1):237–44. [PubMed] [Google Scholar]
- Cheung AT, Ramanujam S, Greer DA, Kumagai LF, Aoki TT. Microvascular abnormalities in the bulbar conjunctiva of patients with type 2 diabetes mellitus. Endocr Pract. 2001;7(5):358–63. doi: 10.4158/EP.7.5.358. [DOI] [PubMed] [Google Scholar]
- Del Corso L, Moruzzo D, Conte B, Agelli M, Romanelli AM, Pastine F, Protti M, Pentimone F, Baggiani G. Tortuosity, kinking, and coiling of the carotid artery: expression of atherosclerosis or aging? Angiology. 1998;49(5):361–71. doi: 10.1177/000331979804900505. [DOI] [PubMed] [Google Scholar]
- Han HC. A biomechanical model of artery buckling. J Biomech. 2007;40(16):3672–8. doi: 10.1016/j.jbiomech.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HC. Nonlinear buckling of blood vessels: a theoretical study. J Biomech. 2008;41(12):2708–13. doi: 10.1016/j.jbiomech.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Han HC. Blood vessel buckling within soft surrounding tissue generates tortuosity. J Biomech. 2009;42(16):2797–2801. doi: 10.1016/j.jbiomech.2009.07.033. [DOI] [PubMed] [Google Scholar]
- Han HC. Determination of the critical buckling pressure of blood vessels using the energy approach. Ann Biomed Eng. 2011;39(3):1032–40. doi: 10.1007/s10439-010-0212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HC. Twisted Blood Vessels: Symptoms, Etiology and Biomechanical Mechanisms. J Vasc Res. 2012;49(3):185–197. doi: 10.1159/000335123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HC, Ku DN, Vito RP. Arterial wall adaptation under elevated longitudinal stretch in organ culture. Ann Biomed Eng. 2003;31(4):403–11. doi: 10.1114/1.1561291. [DOI] [PubMed] [Google Scholar]
- Hayman DM. Biomedical Engineering. San Antonio, TX: University of Texas at San Antonio. PhD; 2011. Effect of Pulse pressure on arterial wall remodeling. [Google Scholar]
- Hayman DM, Xiao Y, Yao Q, Jiang Z, Lindsey ML, Han HC. Alterations in Pulse Pressure Affect Artery Function. Cell & Mol Bioeng. 2012 doi: 10.1007/s12195-012-0251-x. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD. Cardiovascular solid mechanics: cells, tissues, and organs. New York: Springer; 2002. [Google Scholar]
- Jackson ZS, Dajnowiec D, Gotlieb AI, Langille BL. Partial off-loading of longitudinal tension induces arterial tortuosity. Arterioscler Thromb Vasc Biol. 2005;25(5):957–62. doi: 10.1161/01.ATV.0000161277.46464.11. [DOI] [PubMed] [Google Scholar]
- Jakob M, Spasojevic D, Krogmann ON, Wiher H, Hug R, Hess OM. Tortuosity of coronary arteries in chronic pressure and volume overload. Cathet Cardiovasc Diagn. 1996;38(1):25–31. doi: 10.1002/(SICI)1097-0304(199605)38:1<25::AID-CCD7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lee AY. Biomedical Engineering. San Antonio: UTSA. Ph.D; 2011. Determining the Critical Buckling of Blood Vessels Through Modeling and In Vitro Experiments. [Google Scholar]
- Lee AY, Han B, Lamm SD, Fierro CA, Han HC. Effects of elastin degradation and surrounding matrix support on artery stability. Am J Physiol Heart Circ Physiol. 2012;302(4):H873–84. doi: 10.1152/ajpheart.00463.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YU, Hayman D, Sprague EA, Han HC. Effects of Axial Stretch on Cell Proliferation and Intimal Thickness in Arteries in Organ Culture. Cell Mol Bioeng. 2010a;3(3):286–295. doi: 10.1007/s12195-010-0128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YU, Luo J, Sprague E, Han HC. Comparison of artery organ culture and co-culture models for studying endothelial cell migration and its effect on smooth muscle cell proliferation and migration. Ann Biomed Eng. 2010b;38(3):801–12. doi: 10.1007/s10439-009-9877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipzig TJ, Dohrmann GJ. The tortuous or kinked carotid artery: pathogenesis and clinical considerations. A historical review. Surg Neurol. 1986;25(5):478–86. doi: 10.1016/0090-3019(86)90087-x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Han HC. Mechanical buckling of artery under pulsatile pressure. J Biomech. 2012;45(7):1192–8. doi: 10.1016/j.jbiomech.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res. 2011;108(3):365–77. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R, Fierro CA, Shireman PK, Han HC. Mechanical buckling of veins under internal pressure. Ann Biomed Eng. 2010;38(4):1345–53. doi: 10.1007/s10439-010-9929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz H, Murray-Leslie RM, Bannister RG, Bull JW, Marshall J. Kinking of the internal carotid artery. Lancet. 1961;1(7174):424–6. doi: 10.1016/s0140-6736(61)90004-6. [DOI] [PubMed] [Google Scholar]
- Owen CG, Newsom RS, Rudnicka AR, Barman SA, Woodward EG, Ellis TJ. Diabetes and the tortuosity of vessels of the bulbar conjunctiva. Ophthalmology. 2008;115(6):e27–32. doi: 10.1016/j.ophtha.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Pancera P, Ribul M, Presciuttini B, Lechi A. Prevalence of carotid artery kinking in 590 consecutive subjects evaluated by Echocolordoppler. Is there a correlation with arterial hypertension? J Intern Med. 2000;248(1):7–12. doi: 10.1046/j.1365-2796.2000.00611.x. [DOI] [PubMed] [Google Scholar]
- Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, Bochicchio A, Vicinanza C, Aquila I, Curcio A, Condorelli G, Indolfi C. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res. 2011;109(8):880–93. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- Tremblay D, Cartier R, Mongrain R, Leask RL. Regional dependency of the vascular smooth muscle cell contribution to the mechanical properties of the pig ascending aortic tissue. J Biomech. 2010;43(12):2448–51. doi: 10.1016/j.jbiomech.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, McEniery CM. Arterial stiffness, endothelial function and novel pharmacological approaches. Clin Exp Pharmacol Physiol. 2004;31(11):795–9. doi: 10.1111/j.1440-1681.2004.04074.x. [DOI] [PubMed] [Google Scholar]