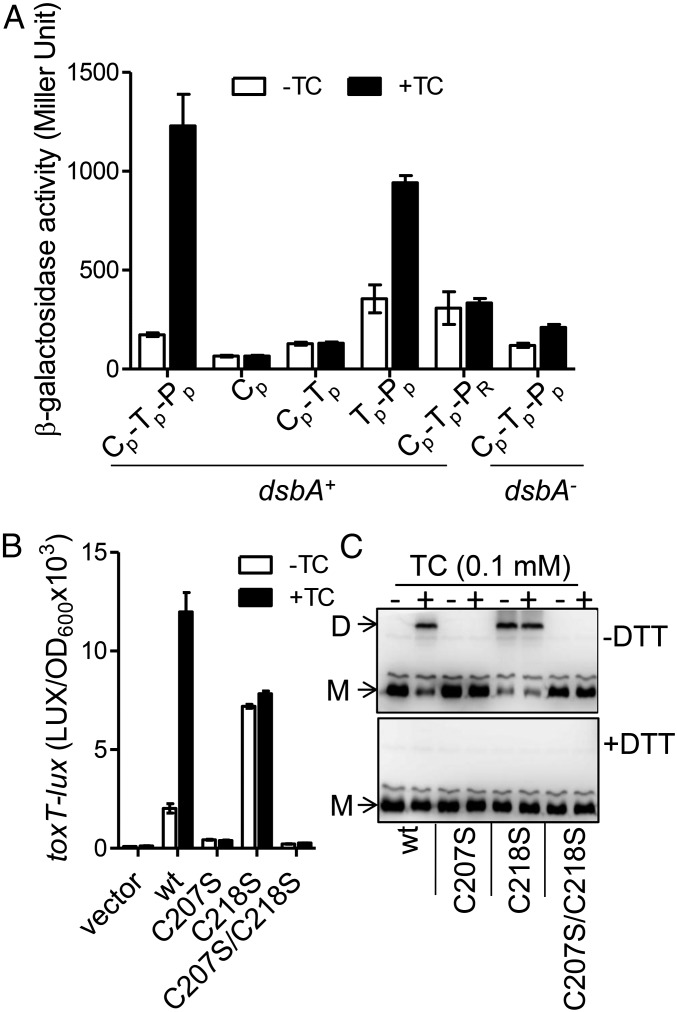
Fig. 3.
Bile salts mediate TcpP dimerization through cysteine-207. (A) Bile salts promote TcpP–TcpP interaction. Full-length TcpP and truncated/chimeric TcpP were fused with the T25 and T18 domains of adenylate cyclase (CyaA) from Bordetella pertussis, respectively, and the T25, T18 fusion pairs were introduced into E. coli cyaA mutants (17) or cyaA/dsbA double mutants (35). Cultures were grown at 30 °C for 8 h and β-galactosidase activity was measured and reported as Miller Units (36). The annotation is the same as in Fig. 2C. (B) V. cholerae ΔtcpPH (pBAD-tcpPH) containing PtoxT-lux reporter and WT or cysteine mutant tcpP under the control of the PBAD promoter on plasmids were grown in LB containing 0.01% arabinose in the absence or in the presence of 100 µM TC until OD600 ≈ 0.2. Luminescence was then measured and reported as light units/OD600. Data are mean and SD of three independent experiments. (C) V. cholerae tcpP deletion mutants containing PBAD-controlled plasmids harboring TcpP and its cysteine mutant derivatives fused with C-terminal FLAG tags were grown in LB containing 0.01% arabinose in the absence or in the presence of 100 µM TC. Then, 1-mg cell lysates were applied to a nonreducing SDS/PAGE (without DTT, Upper) or to a reducing SDS/PAGE (with 10 mM DTT in the loading buffer, Lower), and subjected to the Western blot using anti-FLAG antibody. D, dimer; M, monomer.