Figure 4.
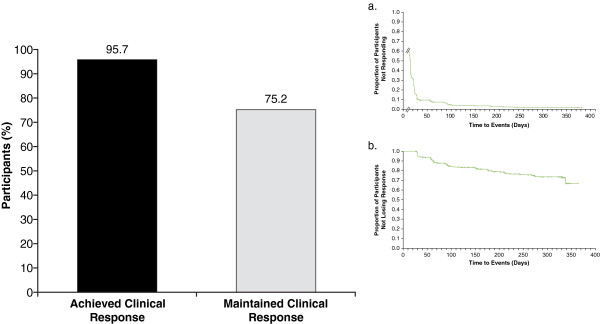
Percentage of participants who achieved clinical response and maintained clinical response and Kaplan-Meier time course (inset) of attainment (a) and loss (b) of clinical response from baseline in the long-term study for all LDX treatment groups. For attainment (a), log rank P-value: .0115; time to first clinical response was calculated from first day of study for participants who entered the maintenance phase of the study (n=327), response status at the start of the long-term study was not determined, and first on-treatment assessment of Attention-Deficit/Hyperactivity Disorder Rating Scale IV and Clinical Global Impressions-Improvement were at week 1; P-value indicates overall significant effect among the treatment groups and was not specifically tested between any individual dose groups. For loss (b), log rank P-value: .5531; time to loss of clinical response during the maintenance phase was calculated for participants who met criteria for response (n=278) at week 4 (visit 5); P-value indicates overall significant effect among the treatment groups and was not specifically tested between any individual dose groups. Abbreviation: LDX=lisdexamfetamine dimesylate.
