Abstract
Objective
Because exogenous estrogen treatment has been associated with a higher risk of urinary incontinence, our objective was to evaluate the longitudinal relationships of dietary phytoestrogen intakes (isoflavones, coumestans and lignans) and the development of incontinence in midlife women transitioning through menopause.
Methods
The Study of Women’s health Across the Nation (SWAN) Phytoestrogen Study was developed within SWAN, a community-based, multisite, multi-racial/ethnic, prospective cohort study. SWAN interviewers administered a food consumption assessment at baseline and at follow-up visits 5 and 9. The SWAN Phytoestrogen study created a phytonutrient data base that allowed estimation of usual daily intakes of four isoflavones, four lignans and coumestrol. On an annual self-administered questionnaire, participants reported on frequency and type of incontinence. We used discrete proportional hazards models to evaluate whether estimated daily intake of each phytoestrogen class at the visit previous to the first report of incontinence was associated with the development of monthly or more incontinence compared to remaining continent.
Results
We found no association or patterns of association between developing any, stress or urge incontinence and the reported daily dietary intake of isoflavones, coumestrol, and lignans in the visit previous to the onset of incontinence.
Conclusions
The results of this longitudinal study provide important information to better understand estrogen-like substances on the continence mechanism in midlife women. Our study shows that neither high nor low dietary intakes of isoflavones, coumestrol and lignans prevent stress or urge incontinence. Future studies should evaluate whether serum levels of phytoestrogens or their metabolites impact incontinence symptoms.
Keywords: Phytoestrogen, isoflavone, coumestrol, lignan, urinary incontinence
Introduction
Exogenous estrogen has been used variably to treat urinary incontinence. While systemic, conjugated equine estrogens are associated with the development and worsening of both stress and urge incontinence in post-menopausal women1–3, other types and routes of estrogens have not been as well studied with mixed results4. Selective estrogen receptor modulators (SERMS) have variable effects on incontinence. For example, testing of levormeloxifene, a SERM with high estrogenic activity on urogenital tissues5–7, was halted in part due to an associated four-fold increased incidence of incontinence, from 4% to 17%8. Additionally, the development of incontinence appeared to be dose related with a higher rate of incontinence among women taking higher levormeloxifine doses9. Meanwhile, raloxifene, a SERM with low estrogenic activity on the urogenital tissues, had no effect on incontinence10. The clinical effect of pharmaceutical estrogens and SERMs on incontinence raises interest in the effects of dietary sources of estrogen receptor modulating compounds11, 12.
Phytoestrogens (“plant estrogens”) are heterocyclic phenols found in many plant foods. Three major categories occur within the general class of phytoestrogens: isoflavones (e.g. daidzein and genistein), coumestans (e.g., coumestrol), and lignans (e.g. secoisolariciresinol and matairesinol). Based on the primary action of these classes on estrogen receptors and the highest level of evidence that the action of systemic estrogens increase the risk of developing incontinence, we hypothesized that isoflavones and coumestrol’s more agonist action would be associated with an increased risk of developing both stress and urge incontinence while lignan’s more antagonist action would reduce the occurrence of both incontinence types13. There is scant data evaluating the effects of individual phytoestrogen classes on incontinence. In one small pilot trial, twelve weeks of a “phytoestrogen deficient diet” was associated with an increased frequency of urge incontinence only while a soy (isoflavone) rich diet did not have an effect on incontinence11. These results encourage further investigation of phytoestrogens on incontinence, but large epidemiological studies are first needed to explore associations between different classes of dietary phytoestrogens and incontinence.
Our objective was to evaluate the longitudinal relationships of dietary intakes of isoflavones, coumestans or lignans, and the development of incontinence in midlife women transitioning through menopause.
Methods
Study sample
The Study of Women’s Health Across the Nation (SWAN) Phytoestrogen Study sample is derived from participants in SWAN, a community-based, multisite, multi-racial/ethnic, prospective cohort study of the menopausal transition (MT) and midlife14. Briefly, entry criteria for the SWAN cohort were: age 42 to 52 years; having at least one ovary and an intact uterus; no current use of estrogens or other medications known to affect ovarian function; having had least one menstrual period in the 3 months prior to screening; and self-identification as white, African American, Hispanic, Chinese, or Japanese. Women in SWAN have been followed for over 10 years at 7 sites. Usual dietary intake data were collected using a food frequency questionnaire (FFQ) administered at baseline and during follow-up visits 5 and 9. In the Phytoestrogen Study, participants from 6 SWAN sites (Boston, Chicago, Detroit, Pittsburgh, Oakland, and Los Angeles [N=2870] were eligible for inclusion. We omitted data from one SWAN site (Newark) because of high attrition (up to 45%) and because that site did not collect any dietary data at follow-up 9. We also excluded participants based on availability of diet data and dietary quality control standards as follows: did not have diet assessment (N = 11); reported intake of less than 4 or greater than 17 solid foods per day (N = 130); skipped more than 10 food items when responding to the FFQ (N = 1); had a calculated daily energy intake of <500 kcal or >5,000 kcal (N = 7). If participants met any of these diet-based exclusionary criteria at later visits, we set their dietary variables to missing for those visits. Thus, the SWAN Phytoestrogen Study sample consists of 2721 women at baseline, 1905 at follow-up visit 5, and 1677 at follow-up visit 9. Inclusion in the current study of phytoestrogens and incontinence required that participants reported no incontinence at baseline so that they could be followed for new reports of incontinence (N=1459 women at baseline, 981 at follow-up visit 5, and 883 at follow-up visit 9).
Phytoestrogen Intake
At baseline and at follow-up visits 5 and 9, SWAN used an interviewer-administered dietary assessment to determine usual food consumption during the past year. The SWAN diet instrument had 3 components: 1) a full food frequency questionnaire (FFQ); 2) an “Ethnic Foods Page”, and 3) open-ended questions. The SWAN FFQs were based on the Block FFQ15 and 4 SWAN versions tailored to language/ethnicity. The English-language version contained a 103-item core food list, based on Second National Health and Nutrition Examination Survey (NHANES II). The Chinese and Japanese ethnic group versions included the same 103-item core food list plus 12 to 16 additional foods appropriate for the each group, called the “Ethnic Foods Pages”. Finally, all women were asked an open-ended question about any other foods eaten at least weekly. The SWAN Phytoestrogen Study created a new phytonutrient data base using all available data through 2008 and computed usual daily intakes of 4 isoflavones, 4 lignans and coumestrol16, 17.
Urinary Incontinence
SWAN obtained baseline and annual information on incontinence symptoms, incontinence frequency and type though a self-administered questionnaire. Based on response to the question: “In the past year (or since your last study visit), have you ever leaked even a small amount of urine involuntarily?”, we classified frequency of incontinence as “almost daily/daily” (daily), “several days per week” (weekly), “less than one day per week” (monthly), “less than once a month” or “none.” We defined any incontinence as incontinence occurring at least monthly. We considered incontinence occurring less than once a month as clinically insignificant and subject to a higher misclassification rate and therefore combined this category with “no incontinence.” We categorized type of incontinence as “stress” if participants reported leakage with “coughing, laughing, sneezing, jogging, jumping, with physical activity or picking up an object from the floor” or as “urge” if participants reported leakage “when you have the urge to void and can’t reach the toilet fast enough.”
Women who were continent at baseline but then reported incontinence at any of the annual follow-up visits were considered to have incident incontinence and were compared to women who did not develop incontinence over the same time frame.
When a woman was missing data on frequency and type of incontinence from one or two visits, we imputed values as follows18, 19. If the missing value occurred at year 10, we imputed the value at the previous visit. If women reported no incontinence in the years previous and subsequent to a missing incontinence report, we assumed no incontinence in those missng years. If a woman was missing incontinence data in the one to two years previous to a first report of incontinence we randomly assigned her missing values to either no incontinence or the frequency and/or type of incontinence in that subsequent year. We imputed incontinence frequency for 1959 observations (6.5% of all observations), and incontinence type for 3207 observations (10.7% of all observations)
Other Co-Variates
Our baseline covariates included baseline age, BMI, diabetes, parity, socioeconomic status, education, number of premenstrual symptoms, as well as symptom sensitivity from the first annual follow up visit. Time dependent co-variates were menopause stage, hormone use, perceived stress, SHBG and E2 level. We also evaluated time-varying covariates from the visit previous (lagged) because we would anticipate incontinence to develop or not develop after the emergence or change in the variable: incident diabetes, hypertension, depressive symptoms and anxiety symptoms, smoking status, weight change (current year value subtracted from previous year value in pounds), number of stressful life events, overall health and caloric intake.
Co-variates were ascertained by the following methods. SWAN classified menopause transition stage annually from menstrual bleeding patterns. Pre-menopause was less than three months of amenorrhea and no menstrual irregularities in the previous year; early peri-menopause was less than three months of amenorrhea and some menstrual irregularities in previous year; late peri-menopause was three to 11 months of amenorrhea; and postmenopause as 12 consecutive months of amenorrhea. We calculated body mass index (BMI) as weight in kilograms/(height in meters)2 based on measurements taken annually by certified staff who used calibrated scales and a stadiometer. Screening socioeconomic status was approximated by level of difficulty paying for basics (food, heat and shelter). Interviewers obtained self-reported medical histories, smoking history and medication use. We considered a woman diabetic if she reported the diagnosis of diabetes or reported the use of diabetic medications. Each year SWAN used the same questions from the Center for Epidemiological Studies-Depression scale20 (we defined depressive symptoms as a score of 16 or above), the Medical Outcomes Study Social Support Survey21, the Life Stressors and Social Resources Inventory22, and the Psychiatric Epidemiology Research Interview23. SWAN measured anxiety symptoms by a summed score of days in the past two weeks in which certain symptoms were experienced (grouchiness or irritability, feeling tense or nervous, pounding or racing heart, feeling fear for no reason); we defined anxiety symptoms by a score of 4 or more24. At year one only, SWAN combined responses to questions assessing sensitivity to physical sensations into a Symptom Sensitivity Scale25. For estradiol (E2), SWAN used a rabbit anti-E2-6 ACS-180 immunoassay with a lower limit of detection of 1.0 pg/mL and conducted duplicate E2 assays with results reported as the arithmetic mean for each participant (coefficient of variation of 3–12%). We adjusted these levels by day of menstrual cycle for women still having periods and sex hormone binding globulin (SHBG).
Phytoestrogen interpolation
SWAN measured study outcomes annually, but dietary exposure variables were measured only at visits 00, 05, and 09. To handle the differences in measurement schedules, dietary variables were interpolated one at a time using random effects modeling as a function of time on study26, after applying a log transformation due to right-skewness. These models were stratified on race, due to large racial/ethnic differences in phytoestrogen consumption. Loess curves indicated linear time trends, thus each model included a random (woman-specific) intercept and slope for time on study. The woman-specific regression coefficients are weighted averages of the coefficients from the full sample and the coefficients from each participant’s data only27. Adding predictors other than time on study did little to improve prediction, and missing predictor data reduced the available sample size.
To assess the performance of these models, we compared fitted values with observed values from visits 00, 05, and 09. Pearson correlations between fitted and observed, accounting for within-woman correlation28 ranged from 0.978 to 0.996, indicating excellent agreement. Additionally, linear regressions of observed values on fitted values indicated no systematic bias, as intercepts were close to 0 and slopes were close to 1. Finally, loess curves for observed values versus time on study overlapped considerably with corresponding curves for fitted values.
We interpolated (i.e., imputed) logged dietary variables for visits at which the FFQ was not administered, using the participant-specific intercept and slope coefficients and the relevant value of time on study for each non-FFQ study visit. Given the high agreement between observed and fitted values, we did not employ multiple imputation. Additionally, we ran two sets of sensitivity analyses: first, including only predictor and outcome data observed at FFQ visits (00, 05, and 09); and second, including all visits in analyses but carrying the last measured dietary variable value forward, which implicitly imputes based on only a single previous observation, in contrast to the model-based imputation based on all of a woman’s observed FFQ data. Results from these sensitivity analyses were similar to those using the interpolated dietary values, except on two occasions. Specifically, there was a statistically significant association in the same positive direction between the second tertile of ligand intake and stress incontinence where there had been none in the non-Asian group and no association between the second tertile of isoflavone intake and stress incontinence in the Asian group where there had been one (data not shown). While these results suggest that some differences in interpolation may affect the significance of our results, the lack of a monotonic association and similar ORs suggest no important effect. Thus we present the interpolated results which we feel to be more representative of phytoestrogen intakes29.
Data analysis
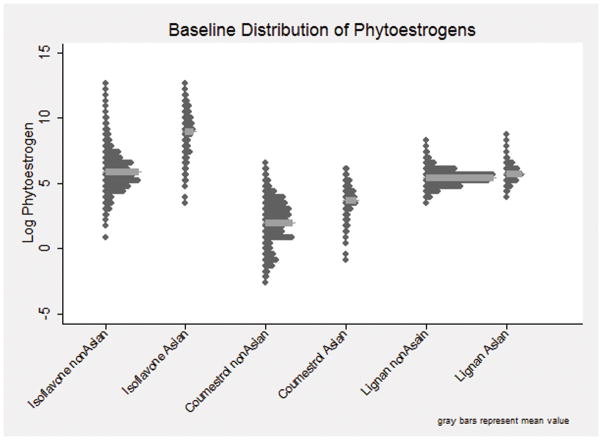
First, we plotted distributions of the logged values of each class of phytoestrogen (total isoflavones, coumestrol, and total lignans) in each racial/ethnic group. We found a mostly bimodal distribution of isoflavones and coumestrol, with different means and distributions between the Chinese and Japanese intakes and the African American and white phytoestrogen intakes. Because these intake distributions had little overlap, we categorized women into Asian (Chinese and Japanese) and non-Asian (white and African American) racial groups for our analyses (Figure 1). For consistency between isoflavones, coumestrol and lignans, and to allow for possible non-linear associations, we categorized each phytoestrogen class based on tertiles in the Asian and non-Asian subgroups and ran relational analyses in stratified samples of Asian and non-Asian women.
Figure 1.
Baseline Distribution of Isoflavone, Coumestrol, and Lignan Intake by Asian and Non-Asian Groups.
See attached TIF file.
We compared proportions and means of each variable at baseline for women who did and did not develop incontinence using the chi-squared and Wilcoxon Sign Rank test for categorical and continuous variables respectively. For baseline phytoestrogen class comparisons, we used the Wilcoxon Sign Rank test due to skewness. We used discrete proportional hazards models30 to evaluate whether phytoestrogen intake at the previous visit was associated with the development of monthly or more incontinence compared with no development of any incontinence at the current visit. For all our analyses, we evaluated phytoestrogen consumption from the previous visit to ensure that the dietary exposures preceded new onset incontinence. We created similar separate models for stress and urge incontinence. For stress incontinence, our comparison group was those women who had no development of stress incontinence and for urge incontinence it was those women who had no development of urge incontinence.
The candidate covariates described above were chosen based on the literature, a priori hypotheses and/or association with the outcome in univariable analysis at p < 0.10. We also adjusted for correlates of the missing FFQ data that reduced our sample size over the study, including ethnicity (stratifying factor), education level, weight, and anxiety, in order to reduce possible nonresponse bias31. We used SAS 9.2, SAS Institute Inc., Cary, NC, USA.
Results
Women who developed incontinence (incident incontinence) differed in several ways from women who remained continent. Those with new onset incontinence had a higher level of education, reported less economic hardship (that is, had an easier time paying for basic necessities), were more likely to have diabetes, a higher BMI and a higher calorie intake, and were more likely to report anxiety symptoms, pre-menstrual symptoms, and life stressors compared to women who never reported incontinence (Table 1).
Table 1.
Baseline Characteristics of SWAN Phytoestrogen Women Who Developed Incident Incontinence and Remained Continent over 10 years of Follow-Up*
| Category | All Women (N=1459) | Incident Incontinence (N=880) | Remained Continent (N=579) | Chi-Squared p-value† | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
|
| |||||||
| Race/ethnicity | |||||||
| African American | 527 | 36.12 | 303 | 57.50 | 224 | 42.50 | 0.025 |
| Caucasian | 622 | 42.63 | 403 | 64.79 | 219 | 35.21 | |
| Chinese | 158 | 10.83 | 87 | 55.06 | 71 | 44.94 | |
| Japanese | 152 | 10.42 | 87 | 57.24 | 65 | 42.76 | |
|
| |||||||
| Education | |||||||
| Less than high school | 66 | 4.56 | 30 | 45.45 | 36 | 54.55 | 0.004 |
| High school equivalent | 255 | 17.61 | 137 | 53.73 | 118 | 46.27 | |
| Some college | 472 | 32.60 | 284 | 60.17 | 188 | 39.83 | |
| College grad | 308 | 21.27 | 192 | 62.34 | 116 | 37.66 | |
| Graduate school | 347 | 23.96 | 228 | 65.71 | 119 | 34.29 | |
|
| |||||||
| Difficulty paying for basics | |||||||
| Not very hard | 932 | 65.31 | 544 | 58.37 | 388 | 41.63 | 0.026 |
| Somewhat hard | 399 | 27.96 | 264 | 66.17 | 135 | 33.83 | |
| Very hard | 96 | 6.73 | 56 | 58.33 | 40 | 41.67 | |
|
| |||||||
| Overall Health | |||||||
| Excellent | 133 | 9.20 | 76 | 57.14 | 57 | 42.86 | 0.350 |
| Very good | 185 | 12.80 | 119 | 64.32 | 66 | 35.68 | |
| Good | 956 | 66.16 | 567 | 59.31 | 389 | 40.69 | |
| Fair | 155 | 10.73 | 101 | 65.16 | 54 | 34.84 | |
| Poor | 16 | 1.11 | 8 | 50.00 | 8 | 50.00 | |
|
| |||||||
| Menopause status | |||||||
| Premenopausal | 815 | 57.11 | 499 | 61.23 | 316 | 38.77 | 0.427 |
| Early Per menopause | 612 | 42.89 | 362 | 59.15 | 250 | 40.85 | |
|
| |||||||
| Parity | |||||||
| Parous | 1160 | 81.01 | 711 | 61.29 | 449 | 38.71 | 0.191 |
| Nulliparous | 272 | 18.99 | 155 | 56.99 | 117 | 43.01 | |
|
| |||||||
| Diabetes | |||||||
| Yes | 46 | 3.16 | 35 | 76.09 | 11 | 23.91 | 0.027 |
| No | 1412 | 96.84 | 845 | 59.84 | 567 | 40.16 | |
|
| |||||||
| Depressive Symptoms | |||||||
| Depressed (16 or more) | 278 | 19.05 | 177 | 63.67 | 101 | 36.33 | 0.204 |
| Not Depressed (<16) | 1181 | 80.95 | 703 | 59.53 | 478 | 40.47 | |
|
| |||||||
| Anxiety symptoms | |||||||
| 4 or less | 1333 | 91.74 | 794 | 59.56 | 539 | 40.44 | 0.039 |
| >4 | 120 | 8.26 | 83 | 69.17 | 37 | 30.83 | |
|
| |||||||
| Premenstrual symptoms | |||||||
| 0–3 symptoms | 587 | 41.25 | 330 | 56.22 | 257 | 43.78 | 0.013 |
| 3–4 symptoms | 836 | 58.75 | 525 | 62.80 | 311 | 37.20 | |
|
| |||||||
| Mean (SD) | Wilcoxon p-value† | ||||||
|
| |||||||
| Age | 45.76 | 2.65 | 45.72 | 2.63 | 45.81 | 2.67 | 0.562 |
|
| |||||||
| BMI | 27.19 | 6.88 | 27.86 | 7.20 | 26.19 | 6.23 | <0.001 |
|
| |||||||
| Perceived stress | 8.18 | 2.86 | 8.38 | 2.83 | 7.88 | 2.88 | 0.001 |
|
| |||||||
| Social Support | 12.64 | 3.08 | 12.52 | 3.02 | 12.83 | 3.16 | 0.007 |
|
| |||||||
|
| |||||||
| Symptom sensitivity | 10.05 | 3.57 | 10.00 | 3.42 | 10.14 | 3.84 | 0.720 |
|
| |||||||
| Stressful Life Events | 3.48 | 2.30 | 3.67 | 2.33 | 3.19 | 2.23 | <0.001 |
|
| |||||||
| Premenstrual symptoms | 0.59 | 0.49 | 0.61 | 0.49 | 0.55 | 0.50 | 0.013 |
|
| |||||||
| Estradiol | 78.25 | 75.55 | 76.58 | 70.59 | 80.78 | 82.51 | 0.855 |
|
| |||||||
| FSH | 24.40 | 26.15 | 23.66 | 26.05 | 25.51 | 26.27 | 0.063 |
|
| |||||||
| SHBG | 47.37 | 25.28 | 45.48 | 23.46 | 50.24 | 27.60 | 0.002 |
|
| |||||||
| Caloric intake | 1833.66 | 691.92 | 1862.49 | 691.57 | 1789.83 | 690.74 | 0.025 |
|
| |||||||
| Fiber intake | 12.53 | 6.06 | 12.49 | 5.77 | 12.58 | 6.47 | 0.812 |
Incident incontinence = reported no incontinence at baseline but developed incontinence in any follow up year, Remained continent = never developed incontinence over the 10 years in SWAN
p-value compares incident and remained continent groups
SD = standard deviation
The mean intakes of isoflavones were about 10-fold lower in the non-Asian compared with the Asian group. Coumestrol intake in non-Asians was about half that of the Asian group, while lignan was only slightly lower (Table 2).
Table 2.
Baseline Dietary Phytoestrogen Intake by Class in Women Who Developed Incontinence and Who Remained Continent Over 10 Years in SWAN*
| Phytoestrogen intake in mcg | Incident Incontinence (N=880) | Remained Continent (N=579) | Wilcoxon p-value | ||
|---|---|---|---|---|---|
| Median | Quartile Range | Median | Quartile Range | ||
|
| |||||
| For Chinese/Japanese | |||||
| Isoflavones | 11512.17 | 24748.64 | 9717.91 | 15265.30 | 0.137 |
| Coumestrol | 45.93 | 71.54 | 39.00 | 56.00 | 0.304 |
| Ligans | 339.41 | 297.70 | 291.43 | 226.70 | 0.076 |
|
| |||||
| For Black/White | |||||
| Isoflavones | 292.01 | 614.30 | 315.60 | 707.26 | 0.559 |
| Coumestrol | 8.17 | 22.90 | 8.09 | 22.19 | 0.405 |
| Ligans | 237.78 | 172.98 | 207.65 | 148.50 | 0.009 |
Incident Incontinence = reported no incontinence at baseline but developed incontinence in any follow up year, Remained continent = never developed incontinence over the 10 years in SWAN
Over the 10 years of observation, the number of women reporting new onset monthly or more incontinence of any type declined (Table 3). The cumulative incidence rate of the entire sample over 10 years for any incontinence was 135 per 1000 person-years. Asian women had a lower cumulative incidence of incontinence (105 per 1000 person-years) compared with non-Asian women (146 per 1000 person-years) with urge incontinence being more common in the non-Asian group (92 per 1000 person-years) compared with the Asian group (53 per 1000 person-years), but stress incontinence being more similar (95 per 1000 person-years in non-Asians versus 92 per 1000 person-years in Asians).
Table 3.
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | Year 7 | Year 8 | Year 9 | Year 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Developed UI | ||||||||||
| Asians | ||||||||||
| Any | 59 | 23 | 27 | 20 | 13 | 9 | 7 | 3 | 5 | 7 |
| Stress | 53 | 31 | 23 | 17 | 10 | 10 | 7 | 5 | 9 | 7 |
| Urge | 34 | 22 | 22 | 20 | 12 | 9 | 6 | 13 | 11 | 13 |
| Non-Asians | ||||||||||
| Any | 274 | 142 | 80 | 61 | 47 | 22 | 30 | 22 | 15 | 13 |
| Stress | 221 | 130 | 89 | 57 | 42 | 24 | 38 | 32 | 21 | 8 |
| Urge | 258 | 146 | 103 | 68 | 44 | 36 | 59 | 33 | 39 | 32 |
Figures in table are the number of women who developed new-onset of urinary incontinence during each year of follow-up.
Definitions of incontinence include: any incontinence = first report of stress, or urge or both in women who reported no incontinence in the previous years, stress incontinence = first report of stress incontinence in women who reported no stress incontinence in the previous years, urge incontinence = first report of urge incontinence in women who reported no urge incontinence in the previous years
In both unadjusted and multivariable models, we found no statistically significant (p<0.05) or consistent patterns of association between developing any incontinence, or specific subtypes (stress or urge incontinence) and the reported dietary intake of isoflavones, coumestrol, or lignans. The exposure to each class of phytoestrogen was estimated based on the visit previous to the reported onset of incontinence (Table 4). While Asian and non-Asian women have different intake ranges of each phytoestrogen class (Figure 1), our results show that within these ranges, dietary phytoestrogens did not prevent or increase the odds of developing incontinence of any type, regardless of menopausal stage.
Table 4.
Adjusted Association between Phytoestrogen Tertiles and Developing Any, Stress or Urge Incontinence between Asians and Non-Asians
| Asians (Japanese and Chinese)* | Non-Asians (African American and white)† | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Type of Phytoestrogen by tertiles (mcg) | Type of Incontinence | OR | 95% CI | Group p- value | Type of Phytoestrogen by tertiles (mcg) | Type of Incontinence | OR | 95% CI | Group p–value |
|
| |||||||||
| Isoflavones | Isoflavones | ||||||||
|
| |||||||||
| 1st tertile ≤ 9.06 | Any | Ref | 0.306 | 1st tertile ≤ 5.50 | Any | Ref | 0.140 | ||
| Stress | 0.017 | Stress | 0.940 | ||||||
| Urge | 0.219 | Urge | 0.773 | ||||||
|
| |||||||||
| 2nd tertile 9.07–9.94 | Any | 0.74 | 0.45, 1.23 | 2nd tertile 5.51–6.29 | Any | 1.02 | 0.80, 1.29 | ||
| Stress | 0.54 | 0.32, 0.91 | Stress | 0.99 | 0.78, 1.25 | ||||
| Urge | 0.68 | 0.43, 1.05 | Urge | 0.93 | 0.75, 1.14 | ||||
|
| |||||||||
| 3rd tertile ≥ 9.95 | Any | 1.11 | 0.67, 1.84 | 3rd tertile ≥ 6.30 | Any | 0.80 | 0.62, 1.04 | ||
| Stress | 1.14 | 0.70, 1.84 | Stress | 0.96 | 0.75, 1.22 | ||||
| Urge | 0.80 | 0.50, 1.29 | Urge | 0.96 | 0.77, 1.19 | ||||
|
| |||||||||
| Coumestrol | Coumestrol | ||||||||
|
| |||||||||
| 1st tertile ≤ 3.36 | Any | Ref | 0.787 | 1st tertile ≤ 1.18 | Any | Ref | 0.522 | ||
| Stress | 0.865 | Stress | 0.625 | ||||||
| Urge | 0.986 | Urge | 0.743 | ||||||
|
| |||||||||
| 2nd tertile 3.37–3.99 | Any | 0.89 | 0.54, 1.47 | 2nd tertile 1.19–2.07 | Any | 0.91 | 0.70, 1.17 | ||
| Stress | 0.93 | 0.58, 1.50 | Stress | 1.13 | 0.88, 1.45 | ||||
| Urge | 0.98 | 0.63, 1.54 | Urge | 1.06 | 0.84, 1.34 | ||||
|
| |||||||||
| 3rd tertile ≥ 4.00 | Any | 1.06 | 0.64, 1.75 | 3rd tertile ≥ 2.08 | Any | 0.87 | 0.68, 1.11 | ||
| Stress | 0.87 | 0.53, 1.43 | Stress | 1.08 | 0.85, 1.37 | ||||
| Urge | 1.02 | 0.65, 1.60 | Urge | 1.09 | 0.87, 1.36 | ||||
|
| |||||||||
| Lignans | Lignans | ||||||||
|
| |||||||||
| 1st tertile ≤ 5.63 | Any | Ref | 0.281 | 1st tertile ≤5.29 | Any | Ref | 0.466 | ||
| Stress | 0.870 | Stress | 0.259 | ||||||
| Urge | 0.185 | Urge | 0.714 | ||||||
|
| |||||||||
|
| |||||||||
| 2nd tertile 5.64–5.98 | Any | 1.26 | 0.75, 2.21 | 2nd tertile 5.30–5.62 | Any | 1.17 | 0.91, 1.50 | ||
| Stress | 0.99 | 0.61, 1.61 | Stress | 1.18 | 0.93, 1.49 | ||||
| Urge | 1.24 | 0.78, 1.98 | Urge | 1.04 | 0.83, 1.30 | ||||
|
| |||||||||
| 3rd tertile ≥ 5.99 | Any | 1.53 | 0.91, 2.59 | 3rd tertile ≥ 5.63 | Any | 1.05 | 0.81, 1.36 | ||
| Stress | 1.12 | 0.68, 1.85 | Stress | 0.99 | 0.77, 1.26 | ||||
| Urge | 1.54 | 0.97, 2.44 | Urge | 0.10 | 0.88, 1.37 | ||||
OR = odds ratio, CI = confidence interval, Ref = reference
All models adjusted for: study time, menopausal status, weight changes, life events, anxiety symptoms, total caloric intake, education level, parity, history of premenstrual symptoms, baseline age, baseline body mass index, symptom sensitivity, sex hormone binding globulin (SHBG), estradiol (E2), day of cycle that serum for E2 and SHBG was obtained.
Asians, number of observations: Any incontinence (N = 1343) Stress incontinence (N = 1524) Urge incontinence (N = 2490)
Non-Asians, number of observations: Any incontinence (N = 3333) Stress incontinence (N = 4810) Urge incontinence (N = 6423)
Discussion
Alpha and beta estrogen receptors (ERs) have been variably identified in urogenital tissues (bladder, urethra, vaginal mucosa, levator ani muscles, and pubo-cervical fascia32–34) and in the central and peripheral nervous systems35. Exogenous estrogen appears to reduce collagen concentration, decrease the cross-linking of collagen,36 and increase the levels of collagen turnover in peri-urethral tissues37–39, which may lead to weakened urethral support and stress incontinence. In animal studies, estrogen increases the collagen to smooth muscle ratio in the bladder wall, increasing mean resting bladder tension and contractility which may impact urge incontinence.40–42 Though the effects on the continence mechanism are unknown, estrogen has a neuromodulation function, increasing sympathetic nerve density in the pelvis and regulating neurotrophins43.
We found no association between the reported dietary intakes of three phytoestrogen classes (isoflavones, coumestrol, or lignans) and developing any type of incontinence in women transitioning through menopause. There are a number of explanations for this null finding. Most likely, while estrogen treatment is associated with developing incontinence, the phytoestrogens we studied may have no discernible effects due to lower binding affinities for the estrogen receptors compared to estradiol13. Additionally, phytoestrogens bind alpha and beta receptors with different affinities and with different agonist and antagonistic properties13, so the balance of this binding variation for isoflavones, coumestrol and lignans in the urogenital tract in midlife women may not produce tissue effects that pre-dispose to stress or urge incontinence.
We chose to evaluate phytoestrogen consumption in the year previous to the first report of monthly or more incontinence to be certain that the dietary isoflavone, coumestrol and lignan intake levels of interest would precede that incident episode. In our interpolation process we found very little with-in woman variation in phytoestrogen intake from year to year, and so those levels were likely similar through the year of the incident incontinence episode. Because the levels of phytoestrogen intake in the SWAN Asian participants had different medians and ranges than the intake in the non-Asian women, we avoided co-linearity between race and phytoestrogen intake by creating stratified models using Asian and non-Asian tertiles. While exposure levels in Asian and non-Asian strata were unequal (ie: the highest isoflavone tertile in non-Asians was roughly equivalent to the lowest in Asians) we did not find any association between phytoestrogen intake and incontinence in either group and so it is unlikely that racial dissimilarities in food sources or other factors are important in our null findings.
Evaluation of phytoestrogen effects on women’s health outcomes have had varied results. About 30–50% of individuals have gut bacteria that metabolize some phytoestrogens into more active serum metabolites, and variation in metabolite production may account for differences in clinical effects of phytoestrogen intake seen across studies of, for example, bone density and hot flushes44. Since our study evaluated dietary intake of phytoestrogens, we could not determine whether different serum concentrations of more active metabolites may prevent or promote the development of incontinence.
Other limitations in our study include the unavoidable measurement error in underestimating phytonutrient intakes and small-area variation in isoflavone content of soy crops can lead to inaccuracies45. Yet the relative rankings of phytonutrient intakes are likely robust and SWAN minimized differential misclassification by designing their FFQs to accommodate mixed dishes and account for ethnic foods. While attrition in longitudinal analyses may have an impact on the generalizability of our results, we included as predictors important factors correlated with attrition to account for possible nonresponse bias.
The incidence of incontinence declined over the time frame of our study. Explanations for this finding include lower incidence in the early post-menopause which coincides with the later years of the study for most women, and the significant reduction in the women at risk for incontinence over the course of the study given how common incontinence is in midlife women as well as cohort attrition in longitudinal analysis. Our incontinence outcomes were self-reported and have the advantage of reflecting womens’ experience of incontinence but the disadvantage of lower precision in diagnosing type (stress and urge) of incontinence. SWAN used the same incontinence questions on an annual basis. These questions were similar to validated questions and those that have been used widely in other incontinence epidemiological studies of incontinence type46, 47. The sensitivity and specificity of self-reported stress and urge incontinence is estimated at 71–85%% and 60–79% respectively in validated questionnaires48, 49.
Conclusion
While epidemiological studies that show no association are often deemed uninteresting, the results of this longitudinal study provide important information to better understand the role of estrogen-like substances on the continence mechanism in mid-life women. Our study has important generalizable public health relevance showing that neither high nor low dietary intakes of isoflavones, coumestrol, or lignans prevent stress or urge incontinence. Before concluding that phytoestrogens have no effect on incontinence, however, future studies should evaluate whether individual variation in phytoestrogen metabolism measured by serum levels of phytoestrogens and/or their metabolites, impact incontinence symptoms.
Acknowledgments
Financial Support
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this article manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. The SWAN Phytoestrogen Ancillary Study was supported by: AG030448
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Sherry Sherman 1994 – present; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
The authors have no conflict of interest to disclose.
References
- 1.Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. Jama. 2005 Feb 23;293(8):935–948. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 2.Grady D, Brown JS, Vittinghoff E, Applegate W, Varner E, Snyder T. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol. 2001 Jan;97(1):116–120. doi: 10.1016/s0029-7844(00)01115-7. [DOI] [PubMed] [Google Scholar]
- 3.Steinauer JE, Waetjen LE, Vittinghoff E, et al. Postmenopausal hormone therapy: does it cause incontinence? Obstet Gynecol. 2005 Nov;106(5):940–945. doi: 10.1097/01.AOG.0000180394.08406.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cody JD, Richardson K, Moehrer B, Hextall A, Glazener CM. Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev. 2009;4:CD001405. doi: 10.1002/14651858.CD001405.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. Jama. 1999 Jun 16;281(23):2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 6.Cohen FJ, Watts S, Shah A, Akers R, Plouffe L., Jr Uterine effects of 3-year raloxifene therapy in postmenopausal women younger than age 60. Obstet Gynecol. 2000 Jan;95(1):104–110. doi: 10.1016/s0029-7844(99)00554-2. [DOI] [PubMed] [Google Scholar]
- 7.Davies GC, Huster WJ, Lu Y, Plouffe L, Jr, Lakshmanan M. Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol. 1999;93(4):558–565. doi: 10.1016/s0029-7844(98)00476-1. [DOI] [PubMed] [Google Scholar]
- 8.Hendrix SL, McNeeley SG. Effect of selective estrogen receptor modulators on reproductive tissues other than endometrium. Ann N Y Acad Sci. 2001 Dec;949:243–250. doi: 10.1111/j.1749-6632.2001.tb04028.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002 Sep;187(3):521–527. doi: 10.1067/mob.2002.123938. [DOI] [PubMed] [Google Scholar]
- 10.Waetjen LE, Brown JS, Modelska K, Blackwell T, Vittinghoff E, Cummings SR. Effect of raloxifene on urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2004 Feb;103(2):261–266. doi: 10.1097/01.AOG.0000109429.67671.d1. [DOI] [PubMed] [Google Scholar]
- 11.Manonai J, Songchitsomboon S, Chanda K, Hong JH, Komindr S. The effect of a soy-rich diet on urogenital atrophy: a randomized, cross-over trial. Maturitas. 2006 May 20;54(2):135–140. doi: 10.1016/j.maturitas.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Tomaszewski J, Adamiak A, Skorupski P, Rzeski W, Rechberger T. Effect of 17 beta-estradiol and phytoestrogen daidzein on the proliferation of pubocervical fascia and skin fibroblasts derived from women suffering from stress urinary incontinence. Ginekol Pol. 2003 Oct;74(10):1410–1414. [PubMed] [Google Scholar]
- 13.Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci. 2004 Jul;80(1):14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 14.Sowers M, Crawford S, Sternfeld B, et al. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. In: Lobo RA, Kelsey JL, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 15.Block G, Thompson F, Hartman A, Larkin F, Guire K. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. Journal of the American Dietician Association. 1992;92:686–693. [PubMed] [Google Scholar]
- 16.Huang MH, Harrison GG, Mohamed MM, et al. Assessing the accuracy of a food frequency questionnaire for estimating usual intake of phytoestrogens. Nutr Cancer. 2000;37(2):145–154. doi: 10.1207/S15327914NC372_5. [DOI] [PubMed] [Google Scholar]
- 17.Huang MH, Norris J, Han W, et al. Development of an updated phytoestrogen database for use with the SWAN food frequency questionnaire: intakes and food sources in a community-based, multiethnic cohort study. Nutr Cancer. 64(2):228–244. doi: 10.1080/01635581.2012.638434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waetjen LE, Feng WY, Ye J, et al. Factors associated with worsening and improving urinary incontinence across the menopausal transition. Obstet Gynecol. 2008 Mar;111(3):667–677. doi: 10.1097/AOG.0b013e31816386ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waetjen LE, Ye J, Feng WY, et al. Association between menopausal transition stages and developing urinary incontinence. Obstet Gynecol. 2009 Nov;114(5):989–998. doi: 10.1097/AOG.0b013e3181bb531a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–283. [Google Scholar]
- 21.Sherbourne CD, Stewart AL. The MOS social support survey. Social Science and Medicine. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 22.Moos RH, Fenn CB, Billings AG, Moos BS. Assessing life stressors and social resources: applications to alcoholic patients. J Subst Abuse. 1988;1(2):135–152. doi: 10.1016/s0899-3289(88)80017-8. [DOI] [PubMed] [Google Scholar]
- 23.Dohrenwend B, Dohrenwend B. Life stress and ilness: Formulation of the issues. New York: Prodist; 1981. [Google Scholar]
- 24.Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004 Jun 15;159(12):1189–1199. doi: 10.1093/aje/kwh168. [DOI] [PubMed] [Google Scholar]
- 25.Barsky AJ, Goodson JD, Lane RS, Cleary PD. The amplification of somatic symptoms. Psychosom Med. 1988 Sep-Oct;50(5):510–519. doi: 10.1097/00006842-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Beydoun MA, Lhotsky A, Wang Y, et al. Association of adiposity status and changes in early to mid-adulthood with incidence of Alzheimer’s disease. Am J Epidemiol. 2008 Nov 15;168(10):1179–1189. doi: 10.1093/aje/kwn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. New York: John Wiley; 2004. [Google Scholar]
- 28.Lipsitz S, Leong T, Ibraham J, Lipschultz S. A partial correlation coefficient and coefficient of determination for multivariate normal repeated measures data. The Statistician. 2001;50(Part 1):87–95. [Google Scholar]
- 29.Greendale GA, Huang MH, Leung K, et al. Dietary phytoestrogen intakes and cognitive function during the menopausal transition: results from the Study of Women’s Health Across the Nation Phytoestrogen Study. Menopause. Mar 12; doi: 10.1097/gme.0b013e318242a654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diggle P, Heagerty P, Liang K, Zeger S. Analysis of Longitudinal Data. 2. Oxford University Press; USA: 2002. [Google Scholar]
- 31.Little RJA, Rubin DB. Statistical analysis with missing data. 2. New York: Wiley; 2002. [Google Scholar]
- 32.Gebhart JB, Rickard DJ, Barrett TJ, et al. Expression of estrogen receptor isoforms alpha and beta messenger RNA in vaginal tissue of premenopausal and postmenopausal women. Am J Obstet Gynecol. 2001 Dec;185(6):1325–1330. doi: 10.1067/mob.2001.119627. discussion 1330–1321. [DOI] [PubMed] [Google Scholar]
- 33.Copas P, Bukovsky A, Asbury B, Elder RF, Caudle MR. Estrogen, progesterone, and androgen receptor expression in levator ani muscle and fascia. J Womens Health Gend Based Med. 2001 Oct;10(8):785–795. doi: 10.1089/15246090152636541. [DOI] [PubMed] [Google Scholar]
- 34.Smith P, Heimer G, Norgren A, Ulmsten U. Steroid hormone receptors in pelvic muscles and ligaments in women. Gynecol Obstet Invest. 1990;30(1):27–30. doi: 10.1159/000293207. [DOI] [PubMed] [Google Scholar]
- 35.Zoubina EV, Smith PG. Expression of estrogen receptors alpha and beta by sympathetic ganglion neurons projecting to the proximal urethra of female rats. J Urol. 2003 Jan;169(1):382–385. doi: 10.1016/S0022-5347(05)64132-8. [DOI] [PubMed] [Google Scholar]
- 36.Keane DP, Sims TJ, Abrams P, Bailey AJ. Analysis of collagen status in premenopausal nulliparous women with genuine stress incontinence. Br J Obstet Gynaecol. 1997 Sep;104(9):994–998. doi: 10.1111/j.1471-0528.1997.tb12055.x. [DOI] [PubMed] [Google Scholar]
- 37.Falconer C, Ekman-Ordeberg G, Ulmsten U, Westergren-Thorsson G, Barchan K, Malmstrom A. Changes in paraurethral connective tissue at menopause are counteracted by estrogen. Maturitas. 1996 Jul;24(3):197–204. doi: 10.1016/s0378-5122(96)82010-x. [DOI] [PubMed] [Google Scholar]
- 38.Falconer C, Ekman-Ordeberg G, Blomgren B, et al. Paraurethral connective tissue in stress-incontinent women after menopause. Acta Obstet Gynecol Scand. 1998 Jan;77(1):95–100. doi: 10.1034/j.1600-0412.1998.770120.x. [DOI] [PubMed] [Google Scholar]
- 39.Jackson S, James M, Abrams P. The effect of oestradiol on vaginal collagen metabolism in postmenopausal women with genuine stress incontinence. Bjog. 2002 Mar;109(3):339–344. doi: 10.1111/j.1471-0528.2002.01052.x. [DOI] [PubMed] [Google Scholar]
- 40.Aikawa K, Sugino T, Matsumoto S, Chichester P, Whitbeck C, Levin RM. The effect of ovariectomy and estradiol on rabbit bladder smooth muscle contraction and morphology. J Urol. 2003 Aug;170(2 Pt 1):634–637. doi: 10.1097/01.ju.0000068723.05004.ca. [DOI] [PubMed] [Google Scholar]
- 41.Sartori MG, Girao MJ, de Jesus Simoes M, Sartori JP, Baracat EC, Rodrigues de Lima G. Quantitative evaluation of collagen and muscle fibers in the lower urinary tract of castrated and under-hormone replacement female rats. Clin Exp Obstet Gynecol. 2001;28(2):92–96. [PubMed] [Google Scholar]
- 42.Fleischmann N, Christ G, Sclafani T, Melman A. The effect of ovariectomy and long-term estrogen replacement on bladder structure and function in the rat. J Urol. 2002 Sep;168(3):1265–1268. doi: 10.1016/S0022-5347(05)64637-X. [DOI] [PubMed] [Google Scholar]
- 43.Zoubina EV, Mize AL, Alper RH, Smith PG. Acute and chronic estrogen supplementation decreases uterine sympathetic innervation in ovariectomized adult virgin rats. Histol Histopathol. 2001 Oct;16(4):989–996. doi: 10.14670/HH-16.989. [DOI] [PubMed] [Google Scholar]
- 44.Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr. 2009 May;89(5):1664S–1667S. doi: 10.3945/ajcn.2009.26736T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SJ, Ahn JK, Kim SH, et al. Variation in isoflavone of soybean cultivars with location and storage duration. J Agric Food Chem. 2003 May 21;51(11):3382–3389. doi: 10.1021/jf0261405. [DOI] [PubMed] [Google Scholar]
- 46.Brown JS, Grady D, Ouslander JG, Herzog AR, Varner RE, Posner SF. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Heart & Estrogen/Progestin Replacement Study (HERS) Research Group. Obstet Gynecol. 1999;94(1):66–70. doi: 10.1016/s0029-7844(99)00263-x. [DOI] [PubMed] [Google Scholar]
- 47.Jackson RA, Vittinghoff E, Kanaya AM, et al. Urinary incontinence in elderly women: findings from the Health, Aging, and Body Composition Study. Obstet Gynecol. 2004 Aug;104(2):301–307. doi: 10.1097/01.AOG.0000133482.20685.d1. [DOI] [PubMed] [Google Scholar]
- 48.Bradley CS, Rovner ES, Morgan MA, et al. A new questionnaire for urinary incontinence diagnosis in women: development and testing. Am J Obstet Gynecol. 2005 Jan;192(1):66–73. doi: 10.1016/j.ajog.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 49.Brown JS, Bradley CS, Subak LL, et al. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006 May 16;144(10):715–723. doi: 10.7326/0003-4819-144-10-200605160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]