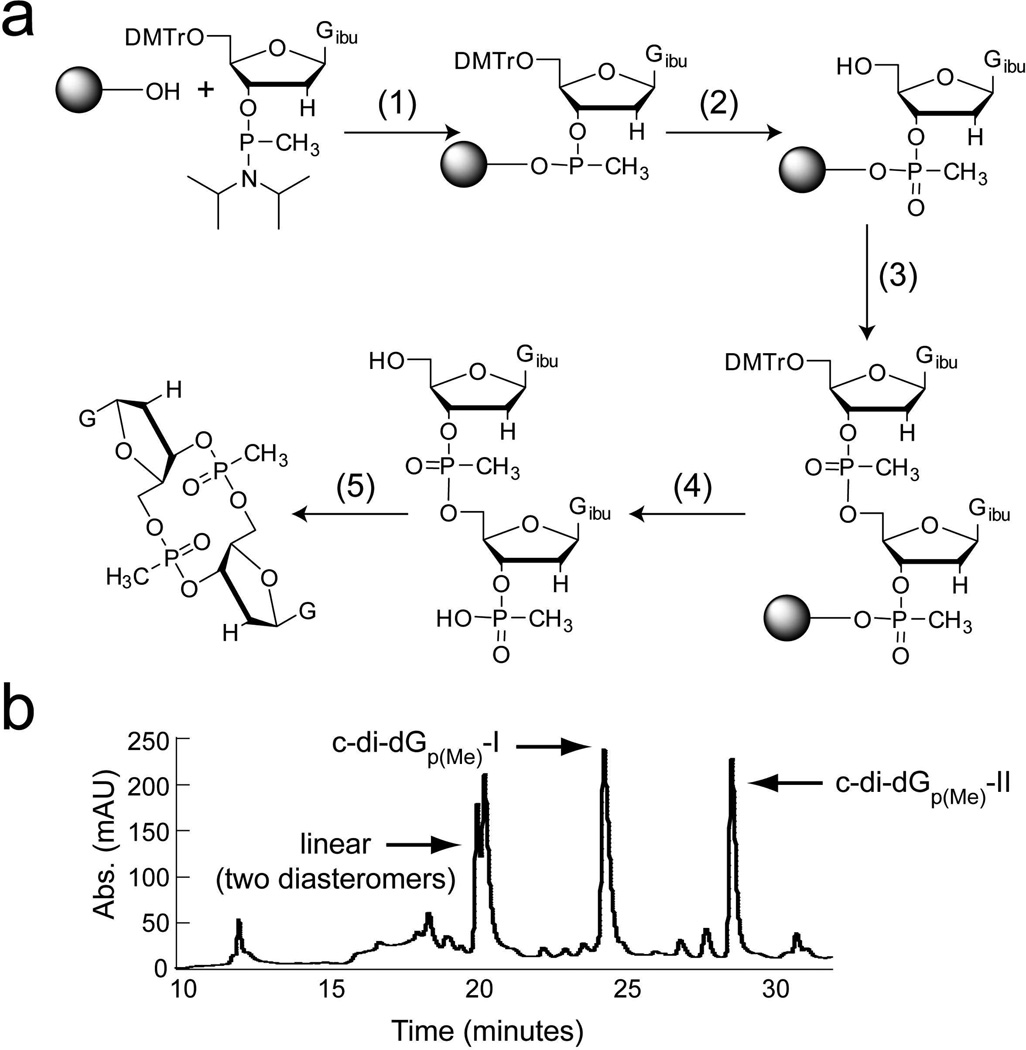
Figure 2.
Synthesis of the double methylphosphonate c-di-GMP analog. (a) Synthetic scheme. The linear dinucleotide was synthesized on solid support and then cyclized in solution. (1) (i) tetrazole/ACN; (2) (i) 3% DCA/DCM (ii) I2/THF/pyridine, (iii) acetic anhydride/DMAP, (iv) 3% DCA/DCM; (3) tetrazole/ACN + guanosine methylphosphonamidite/THF; (4) (i) 3% DCA/DCM, (ii) 10% TEA/ACN; (5) (i) 0.1 M MSNT/pyridine, (ii) ACN/EtOH/NH4OH, (iii) ethylenediamine.
(b) HPLC trace monitored at 254 nm showing purification of the crude reaction synthesis for the methylphosphonate c-di-GMP analog c-di-dGp(Me). Peaks corresponding to the two cyclized diastereomers and the linear, uncyclized molecule are labeled.