Abstract
An 18-month-old female status post-orthotopic liver transplant for biliary atresia presented nine months after transplant with severe diarrhea and intolerance of feeds. She was found to have a PLE as evidenced by a low serum albumin and a persistent elevation of fecal A1AT. Investigation eventually revealed that the cause of the PLE was a stricture at the anastomosis site between the hepatic vein and inferior cava, supported by resolution of the PLE after venoplasty of the stricture. The patient has subsequently required several repeat venoplasties for recurrence of her symptoms correlating with recurrence of the stricture. This is a very rare presentation of hepatic venous outflow obstruction. Moreover, normal duplex ultrasound imaging of liver vasculature and her unusual presentation led to a delay in her diagnosis highlighting the need for an increased index of suspicion.
Keywords: diarrhea, infant, liver transplant
A nine-month-old female infant underwent a successful cadaveric orthotopic left lateral segment liver transplant for end-stage liver failure owing to extrahepatic biliary atresia; she had not previously had a Kasai procedure because of late presentation at four months of age. Her postoperative course was complicated by poor oral intake for which she was supplemented with nasogastric feeds, and a clinical diagnosis of milk protein allergy and mild diarrhea. Approximately nine months after liver transplantation, she presented with a history of increasing diarrhea, intolerance of feeds, and abdominal distension. Physical examination revealed an increase in weight of two kilograms, abdominal distention with ascites, and hepatomegaly; there was no palpable spleen or peripheral lymphadenopathy.
Laboratory investigations at the time were remarkable for hypoalbuminemia with a serum albumin of 1.8 g/dL. Liver and renal function tests were normal. Serum immunoglobulins (IgG, IgA, and IgM) were within the normal ranges suggesting a selective loss of albumin. There was no significant proteinuria. Stool was negative for bacteria, Clostridium difficile toxin, ova and parasites, and enteric viruses. PLE was confirmed by persistent elevation of fecal A1AT, with a maximum value of 960 mg/dL (normal <55 mg/dL). A hepatic duplex ultrasound demonstrated gross ascites and patent hepatic vasculature without evidence of splenomegaly. A CT scan of the abdomen and the pelvis revealed stable lymphadenopathy. An echocardiogram was unremarkable.
Upper gastrointestinal endoscopy revealed grade 1 esophageal varices, unremarkable mucosal biopsies, no lymphangiectasia, and normal disaccharidase assays. Colonoscopy was unremarkable as were multiple colonic mucosal biopsies. An endocrinology workup was normal, including normal thyroid function tests (TSH 2.81 uIU/mL), serum gastrin, 24-hour urine catecholamines, and urine 5-HIAA. The cause of the patient’s presentation at this point was unclear but the diarrhea and dehydration gradually became worse. She was dependent on parenteral nutrition and albumin infusions to maintain stable hemodynamical status. The patient also had persistently positive tests for Epstein-Barr virus in plasma by polymerase chain reaction; however, subsequent investigations including bone marrow biopsy, enteroscopy, and liver biopsy demonstrated no evidence of post-transplant lymphoproliferative disorder. Because of ongoing symptoms of diarrhea and abdominal distention, further investigations were conducted. An enteroscopy was done that showed grade 2 esophageal varices, but was otherwise unremarkable including showing no intestinal lymphangiectasia. A liver biopsy conducted at the same time revealed marked zone 3 sinusoidal congestion and significant centrilobular hepatocyte dropout (Fig. 1) raising the possibility of vascular outflow abnormalities. To investigate this possibility, a transjugular hepatic venogram was conducted. Because the hepatic vein could not be accessed from the transjugular approach, percutaneous access into a peripheral hepatic vein was achieved, and a stricture at the anastomosis site between the hepatic vein-inferior cava was identified (Fig. 2) with HV-RA pressure gradient being 18 mmHg (normal 5 mmHg or less); balloon venoplasty reduced the HV-RA gradient to 4 mmHg. There was no improvement in symptoms at one wk after the venoplasty; therefore, a repeat venogram was conducted which showed marked stenosis at the same site, once again with a reduction in HV-RA gradient to 5 mmHg after balloon venoplasty.
Fig. 1.
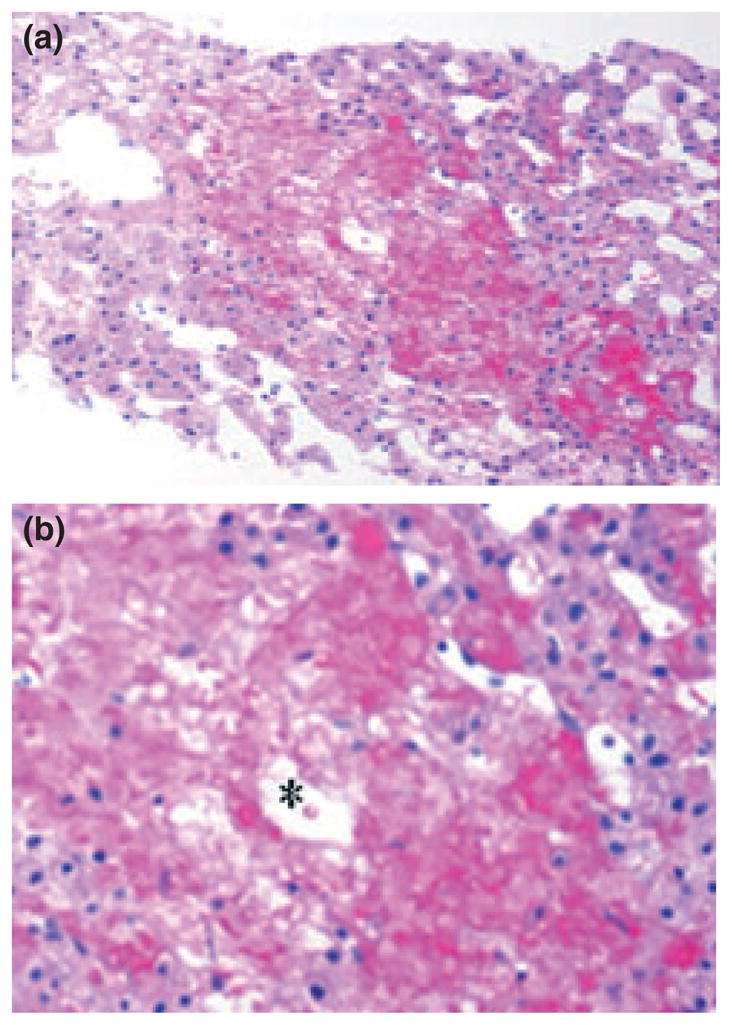
Liver biopsy showing coagulative necrosis around the central vein (*) and sinusoidal dilatation. Original photomicrographs obtained with 4× (a) and 20× (b) objectives.
Fig. 2.
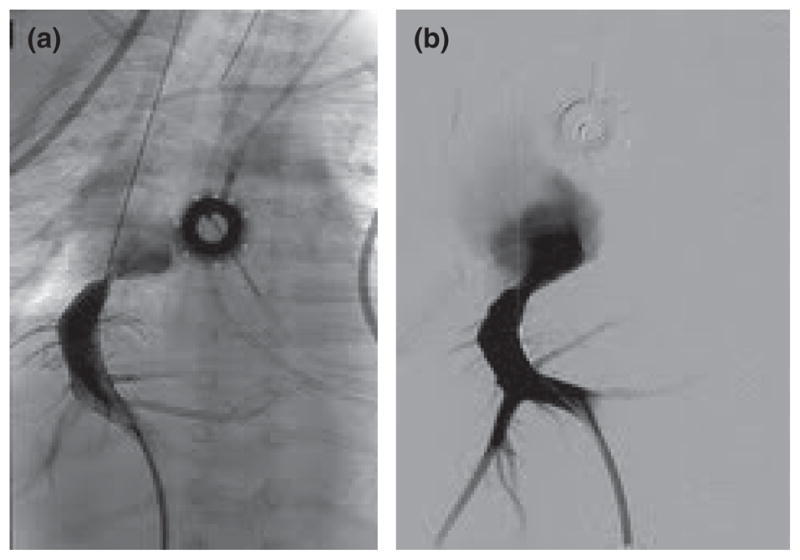
Venogram pre-venoplasty (a) and post-venoplasty (b).
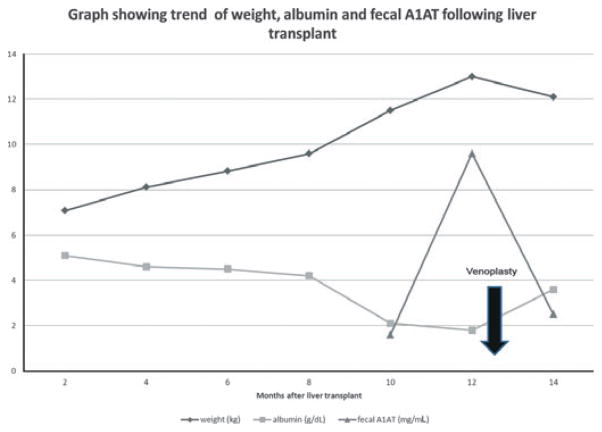
Immediately after the second session of balloon venoplasty, the diarrhea and ascites improved. She was able to tolerate enteral feeds and parenteral nutrition was discontinued. Her serum albumin and fecal A1AT normalized (Fig. 3) and she was discharged from hospital. However, within two months she began to have diarrhea again, with hypoalbuminemia and re-accumulation of ascitic fluid. Repeat venoplasty with reduction of the HV-RA gradient was performed which once again led to an improvement in the patient’s symptoms. Recurrence of persistent diarrhea and ascites was an indication for a total of six sessions of balloon venoplasty in this case.
Fig. 3.
Chart showing the trend of weight, albumin and fecal A1AT after liver transplantation and venoplasty.
Discussion
Vascular complications are a well-known complication of liver transplantation, especially in children, being most common in the portal vein and hepatic artery (1, 2). HVOO following liver transplantation is less common, with an incidence reported between 1% and 7% (3). This often occurs in the early post-operative period (2), possibly from direct compression of the vein, but can occur several months to years later (1), from fibrotic changes at the anastomotic site (3).
In one study, ascites was the most common clinical sign of HVOO following pediatric liver transplantation (4). PLE is a very rare complication of Budd–Chiari syndrome (5), even with inferior vena cava obstruction (6). To our knowledge, there have only been two reported cases of PLE secondary to HVOO following liver transplantation, one in a 42-yr-old male (7), and the other in a 14-yr-old female (8); a case in an infant has not been previously reported.
PLE is caused by abnormalities of the lymphatic system resulting in leakage of protein-rich lymph or mucosal injury with increased permeability (9). It has been hypothesized that in Budd–Chiari syndrome, portal hypertension resulting from the obstruction can lead to bowel mucosa edema, with increased permeability and leakage of albumin, which defines PLE (6). Another theory is the development of acquired lymphangiectasia after liver transplantation (5). In our patient, there was no histologic evidence of intestinal lymphangiectasia on small bowel biopsies and additionally she did not have lymphopenia and low immunoglobulin levels, which can be seen in intestinal lymphangiectasia. However, her presentation was consistent with portal hypertension with gross ascites and esophageal varices seen on endoscopy. One puzzling aspect was that despite evidence of portal hypertension our patient did not develop splenomegaly at any point. In addition, there was a clear temporal relationship between the post-venoplasty decrease in HV-RA pressure gradient and an improvement of diarrhea, serum albumin level, and ascites.
Duplex ultrasound is considered a useful, readily available tool to identify HVOO (10), with findings such as sluggish flow, absence of flow, or reversed flow in outflow veins (4). In our patient, the normal duplex ultrasound of hepatic vasculature and unusual clinical features led to a delay in diagnosis. The duplex ultrasound may have been technically difficult in our patient owing to her young age and agitation. A CT scan of her abdomen and echocardiogram also failed to identify HVOO. After venography, all previous imaging was re-reviewed to look for signs that may have helped detect the HVOO, but none could be identified. Finally, the liver biopsy with zone 3 sinusoidal congestion suggested the diagnosis which was only confirmed by venography.
Treatment of HVOO can be accomplished by open surgical revision, venoplasty, or endovascular stent. Venoplasty is frequently successful but often requires re-intervention (3). Our patient underwent successful venoplasty of the hepatic vein–inferior vena cava anastomosis but has required several repeat procedures. Usually if repeated procedures are needed, an endovascular stent may prove to be beneficial (3). However, in our patient a stent may not be possible owing to the very close proximity of the hepatic vein, inferior vena cava, and right atrium, because of the patient’s small size and location of the anastomosis; therefore, an open surgical revision may be an alternative in the future.
In conclusion, PLE is a very rare presentation of HVOO following liver transplantation. This case highlights the need for an increased index of suspicion especially given the unusual presentation and normal duplex ultrasound findings here leading to delayed diagnosis. Management with venoplasty can be effective but repeated intervention may be necessary. The case also raises the question of whether a patient such as this should be screened regularly with venography to identify recurrence of the stenosis at the anastomotic site as duplex ultrasound was always unrevealing or whether it should be done based on symptomatology. If there are many further recurrences, this patient may eventually benefit from open surgical revision.
Abbreviations
- 5-HIAA
5-hydroxyindoleacetic acid
- CT
computerized tomography
- fecal A1AT
fecal alpha-1 antitrypsin
- HVOO
hepatic vein outflow obstruction
- HV-RA
hepatic vein–right atrium
- PLE
protein-losing enteropathy
- TSH
thyroid stimulating hormone
References
- 1.Buell JF, Funaki B, Cronin DC, et al. Long-term venous complications after full-size and segmental pediatric liver transplantation. Ann Surg. 2002;236:658–666. doi: 10.1097/00000658-200211000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sieders E, Peeters PM, Tenvergert EM, et al. Early vascular complications after pediatric liver transplantation. Liver Transpl. 2000;6:326–332. doi: 10.1053/lv.2000.6146. [DOI] [PubMed] [Google Scholar]
- 3.Wang SL, Sze DY, Busque S, et al. Treatment of hepatic venous outflow obstruction after piggyback liver transplantation. Radiology. 2005;236:352–359. doi: 10.1148/radiol.2361040327. [DOI] [PubMed] [Google Scholar]
- 4.Krishna Kumar G, Sharif K, Mayer D, et al. Hepatic venous outflow obstruction in paediatric liver transplantation. Pediatr Surg Int. 2010;26:423–425. doi: 10.1007/s00383-010-2564-y. [DOI] [PubMed] [Google Scholar]
- 5.Tsuchiya M, Oshio C, Asakura H, Ishii H, Aoki I, Miyairi M. Budd–Chiari syndrome associated with protein-losing enteropathy. Gastroenterology. 1978;75:114–117. [PubMed] [Google Scholar]
- 6.Lee WS, John P, McKiernan P, de Ville De Goyet J, Kelly DA. Inferior vena cava occlusion and protein-losing enteropathy after liver transplantation in children. J Pediatr Gastroenterol Nutr. 2002;34:413–416. doi: 10.1097/00005176-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Dousset B, Legmann P, Soubrane O, et al. Protein-losing enteropathy secondary to hepatic venous outflow obstruction after liver transplantation. J Hepatol. 1997;27:206–210. doi: 10.1016/s0168-8278(97)80303-0. [DOI] [PubMed] [Google Scholar]
- 8.Wong J, Sin NC, Sung R, Lee PS, Lee KF, Lai PB. Budd–Chiari-induced protein-losing enteropathy after liver transplantation. Transplant Proc. 2007;39:1554–1557. doi: 10.1016/j.transproceed.2007.02.095. [DOI] [PubMed] [Google Scholar]
- 9.Braamskamp MJ, Dolman KM, Tabbers MM. Clinical practice. Protein-losing enteropathy in children. Eur J Pediatr. 2010;169:1179–1185. doi: 10.1007/s00431-010-1235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang TL, Chen TY, Chen CL, et al. Hepatic outflow insults in living-related liver transplantation: By Doppler sonography. Transplant Proc. 2001;33:3464–3465. doi: 10.1016/s0041-1345(01)02492-7. [DOI] [PubMed] [Google Scholar]