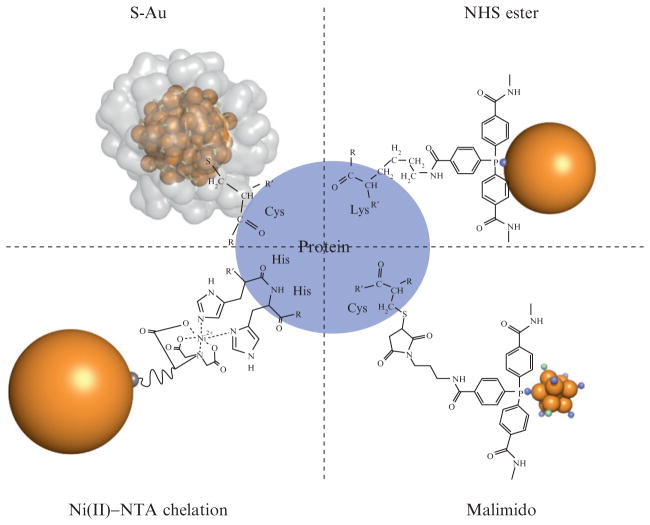
Figure 9.2.
Schematic of the bioconjugation chemistries presented in this chapter, which are summarized in Table 9.1. In the upper left corner is shown a “direct” biomolecule/gold cluster conjugate where a thiol moiety such as a cysteine residue bonds directly to the gold cluster core of Au102(p-mercaptobenzoic acid)44 through a sulfur–gold bond. The crystallographically determined ligand layer of the cluster is rendered as a partially transparent surface in that image, illustrating the steric constraint of this linkage. Shown in upper and lower right panels are the bioconjugates formed by NHS and malimido derivatives of tris (aryl) ligands which protect clusters such as Nanogold and undecagold. Shown at lower left is an Ni2+-mediated interaction between a polyhistidine tag and a nitroloacetic acid (NTA)-derivatized gold cluster. Note that all stable water-soluble gold clusters and colloids have a full organic ligand shell like that shown for Au102 in this diagram, but these ligand layers are sometimes not as well characterized as the cluster core.