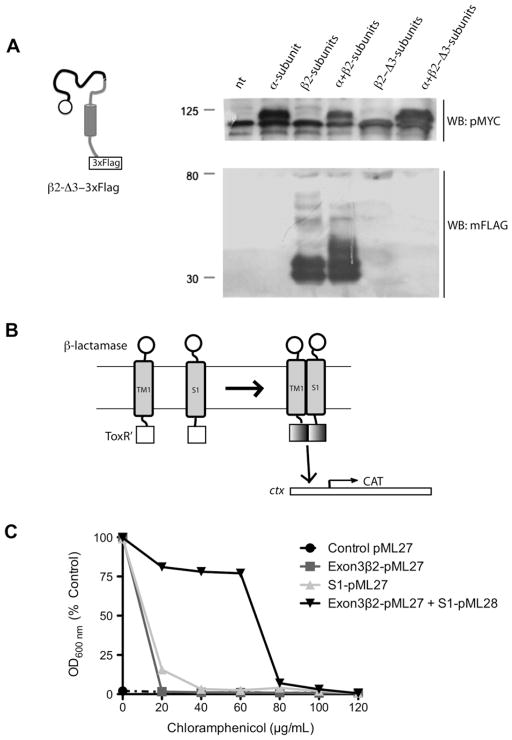
Fig. 4.
The TM1 domain of β2-subunit binds to the S1 domain of α-subunit: (A) Schematic of the β2-Δ3-3×FLAG mutant and Western blot to detect its expression. β2-Δ3-3×FLAG was not detected (N = 2). (B) Schematic representation of TOXCAT assay system. TOXCAT has been designed to measure oligomerization of transmembrane segments of proteins within the context of the cytoplasmic membrane of living cells. In TOXCAT constructs, the transmembrane and periplasmic domains of ToxR were replaced by region coded by exon 3 of β2-subunit (that include TM1 and a short cytoplasmic tail) and S1 of α-subunit and β-lactamase sequences, respectively [21,22]. As a result, dimerization must be driven solely by interactions between the TM segments. Such dimerization allows the cytoplasmic transcriptional activation domains (ToxR′) to interact with the ctx promoter (cholera toxin promoter or ctx is positively regulated by ToxR [27]), thereby initiating transcription of a reporter gene encoding chloramphenicol acetyltransferase (CAT) [21]. TM-induced dimerization is observed as acquired resistance to the antibiotic chloramphenicol in vivo. (C) TOXCAT assay showing dimerization between TM1 (exon3-β2) and S1 (black inverted triangle). Note that bacteria co-transformed with TM1 (exon3-β2) and S1 acquired resistance to chloramphenicol in contrast to bacteria transformed with TM1 (dark gray square) or S1 alone (light gray triangle) (N = 2).