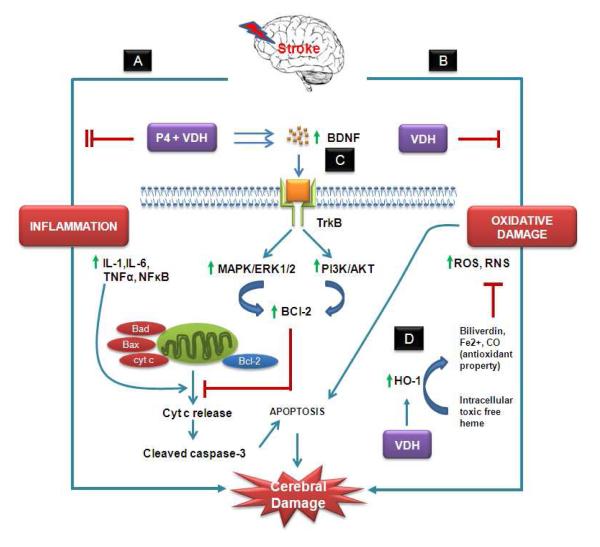
Fig. 7.
Hypothetical presentation of some possible mechanisms of action of P4 and VDH against ischemic stroke. We target two important detrimental pathways of ischemic injury: (A) inflammation, and (B) oxidative damage, and their modulation by P4 and VDH alone and in combination. P4 and VDH inhibited inflammatory response synergistically by suppressing IL-6 and NFκB. Both inflammation and oxidative damage cause mitochondrial damage. As a result, damaged mitochondria release cytochrome c (cyt c) into the cytosol, which further activates caspases and associated apoptosis. (C) Growth factor signaling (BDNF/TrkB/Erk1/2) is one of the neuroprotective mechanisms of P4 and VDH. BDNF promotes cell survival through activation of TrkB, a high-affinity receptor for BDNF. Upon activation, TrkB receptor signaling activates several signaling proteins and pathways regulated by MAP kinase (MAPK/Erk1/2) and PI3 kinase (PI3K/Akt). These pathways further activate the anti-apoptotic protein BCl-2, which prevents mitochondrial damage and cyt-c release-mediated caspase activation and apoptosis, thus promoting neuronal survival. Both P4 and VDH synergistically activate BDNF, TrkB, Erk1/2 signaling, and BCl-2, and suppress cleaved caspase-3 activation. VDH alone showed modulatory effects on oxidative damage as evidenced by the expression of HO-1. HO-1 is a rapidly induced heat shock protein which catabolizes free heme to biliverdin, CO, and Fe2+. These byproducts of HO-1 activity have potential antioxidant activity and provide cytoprotection (D).