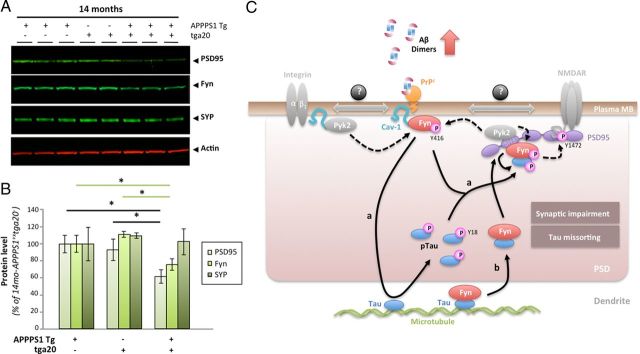
Figure 10.
PrPc overexpression in aged APPPS1 mice triggers a selective decrease in postsynaptic proteins. A, Apparent selective decrease in postsynaptic proteins PSD95 and Fyn in 14-month-old APPPS1+×tga20+ mice. Representative WBs for the postsynaptic proteins PSD95 and Fyn as well as for the presynaptic vesicle protein synaptophysin (SYP) are shown. Actin was used as internal standard. B, Quantification of PSD95, Fyn, and Synaptophysin in 14-month-old APPPS1+×tga20−, APPPS1−×tga20+, and APPPS1+×tga20+ mice revealed significant reductions in postsynaptic proteins in APPPS1 mice overexpressing PrPc (bars represent the mean ± SD; *p < 0.05, ANOVA followed by Student's t test; n ≥ 3 animals/genotype/age/experiment). C, Proposed model of tau regulation by the triad oAβ-PrPc-Fyn. In the presence of accumulating Aβ dimers, PrPc, Fyn, and Cav-1 form a complex at the plasma membrane (likely with receptors such GluN or integrins known to interact with Cav-1 and Fyn). Upon phosphorylation of Fyn at Y416 (possibly by the kinase Pyk2), this complex becomes biologically active. Two scenarios are possible depending on the status of Fyn with respect to tau: Fyn is not bound to tau (a), and Fyn is bound to tau (b). In a, activated Fyn causes the hyperphosphorylation of tau at Y18 and its aberrant accumulation at the postsynaptic density (PSD). It is possible that pFyn might be bound to pTau at this stage and binds the SH3 domain of PSD95, where it is ideally located to modulate GluN/NMDA receptor subunits (i.e., GluN2B at Y1472). In model b, Fyn is already bound to tau in the dendrite, translocate to the PSD to interact with PSD95. There, Pyk2 could phosphorylate Fyn at Y416, resulting in Fyn activation and tau phosphorylation at Y18.