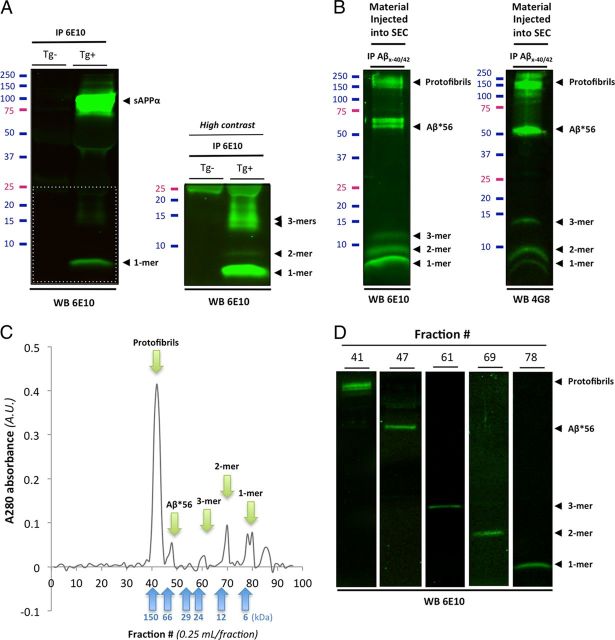
Figure 4.
Biochemical characterization of soluble Aβ species present in Tg2576 conditioned medium and in human brain tissues. Western blot analyses of affinity-purified soluble Aβ species present in conditioned media (CM) of Tg2576 primary cortical neurons and in soluble TBS extracts human AD brain. A, Immunoprecipitation of soluble APP/Aβ molecules present in CM of Tg2576 primary neurons (DIV12–14) using 6E10. A high-intensity laser scan is also shown to better visualize Aβ monomers, dimers, and trimers (bottom inset). B, Representative profile of soluble Aβ oligomers affinity-purified with 40- and 42-end specific antibodies (Mab 2.1.3 and Mab13.1.1; kindly provided by Dr. Pritam Das) to prevent capturing APP molecules and detected with 6E10 (left panel) or 4G8 (right panel). Several soluble Aβ assemblies are readily observed: monomers, dimers, trimers, Aβ*56, a larger 60 kDa oligomer, and protofibrils >150 kDa. C, Typical size exclusion chromatogram of the endogenous human Aβ oligomers shown in B using a Tricorn Superdex 75 column. Five peaks (green arrows) corresponding to the five Aβ species seen by Western blot are clearly observed with elutions at predicted molecular weights (blue arrows correspond to the six molecular weight standards used). D, Representative Western blot images of the respective size exclusion chromatography fractions isolated in C using 6E10.