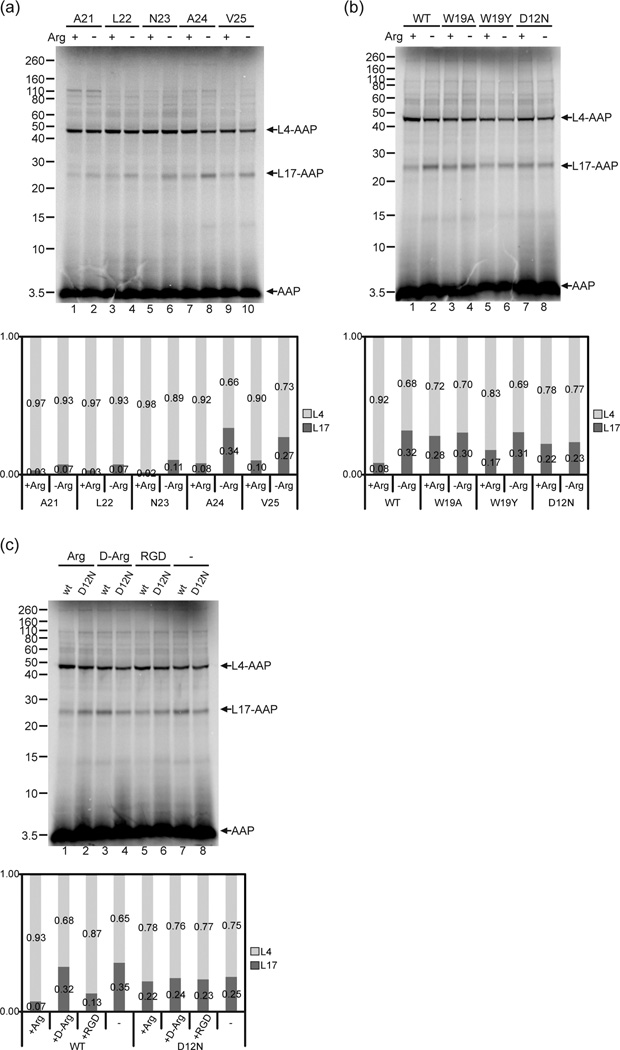
Fig. 6. The change in AAP’s relative conformation detected by photocrosslinking is specifically induced by Arg and requires an AAP sequence that is functional for stalling.
(a) The Arg-induced change in relative conformation occurred when the AAP was shortened at its C-terminus. AAP chains of different lengths with a single εANB-Lys probe at position Val-7 were synthesized in wheat germ extracts in 2 mM Arg (+) or 30 µM Arg (−). Truncation of mRNA was after Ala-21, Leu-22, Asn-23, Ala-24 or Val-25. RNCs were photolyzed and analyzed as in Fig. 2. (b) The Arg-dependent conformational change is related to the AAP’s ability to stall the ribosome. Wild-type (WT), W19A, W19Y, and D12N AAP nascent chains truncated after Ala-24 with a single εANB-Lys probe at position Val-7 were synthesized in 2 mM Arg (+) or 30 µM Arg (−). RNCs were photolyzed and analyzed as in Fig. 2. (c) The Arg-dependent change in photocrosslinking is not a nonspecific electrostatic effect. WT and D12N AAP nascent chains truncated after Ala-24 with a single εANB-Lys probe at position Val-7 were synthesized in the presence of 2 mM L-Arg, D-Arg, or RGD (Arg-Gly-Asp tripeptide), or 30 µM Arg (−). RNCs were photolyzed and analyzed as in Fig. 2. Under each gel image, quantitative analyses of the crosslink products show the relative amount of radiolabel in bands representing AAP crosslinked to L4 and L17 indicated as the fraction of the sum of both crosslink products. Complete quantification data are provided in Supplementary Table 3.