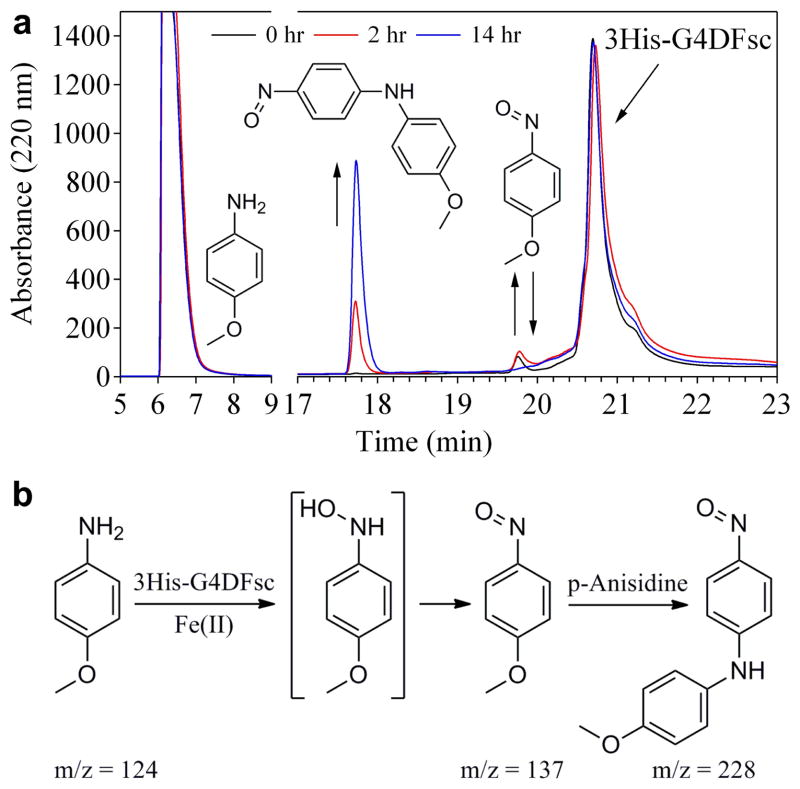
Figure 6.
N-hydroxylation of p-anisidine by 3His-G4DFsc. (a) HPLC chromatograms (monitoring Abs at 220 nm) for the reaction mixture of 0.25 mM 3His-G4DFsc with 5 mM p-anisidine at 0 (black), 2 (red), and 14 (blue) hours after mixing. Products were identified by LC-MS/MS (m/z values indicated in panel b) and comparison with authentic samples. (b) Proposed reaction scheme depicting the oxidation of p-anisidine to p-nitrosoanisole and subsequent nucleophilic aromatic substitution with unreacted p-anisidine to form 4-nitrosodiphenylamine that gives rise to a visible absorption features at 445 nm.