Abstract
Although the histologic changes seen in psoriasis have long been well characterized, the underlying cellular and molecular mechanisms have only begun to be elucidated over the past 20 years. Proinflammatory factors such as tumor necrosis factor (TNF)-α have a central role in psoriasis pathogenesis, and many T helper 1 (Th1) cytokines and messenger RNAs are elevated in psoriatic lesions. Interleukin-17A (IL-17A), IL-17F, and other Th17 cell–derived cytokines have been shown in murine models to induce features that mimic human psoriasis. This review focuses on the emerging biology of the IL-17 cytokine family in psoriasis, and on the molecular and genetic information gained from animal models and human clinical studies that confirm IL-17 as a crucial proinflammatory cytokine in psoriasis. Expression of IL-17A, IL-17C, and IL-17F is strikingly increased in psoriatic lesions, and successful therapy is associated with restoration of the expression of a wide range of genes (including effector molecules downstream of IL-17 such as cytokines, chemokines, and antimicrobial peptides) to near normal levels. Therapeutic agents in development that target IL-17 are discussed, and an emerging model of the key role of IL-17 in the pathogenesis of psoriasis is presented.
Introduction
Psoriasis is a chronic debilitating disease, affecting 1% to 2% of the Caucasian population (Gudjonsson and Elder, 2007; Naldi, 2004) and characterized by recurrent episodes of red and scaly well-demarcated skin plaques (Schon and Boehncke, 2005). The histological changes observed within lesional skin include (1) a thickened epidermis from rapid keratinocyte proliferation and aberrant differentiation, (2) a reduced or absent granular layer, (3) marked dilatation of blood vessels in the papillary dermis, and (4) dense clusters of inflammatory cells composed of T cells and dendritic cells in the dermis, and CD8+ T cells and neutrophils in the epidermis (Schon and Boehncke, 2005). Histologic abnormalities in psoriasis have been well described for decades, and include multiple elements that indicate immune-mediated inflammation. However, the cellular and molecular mechanisms underlying the pathophysiologic changes have only recently been brought into sharp focus by studies describing global alterations in the psoriasis lesional transcriptome, as well as the impressive success of targeted therapeutics in the clinic.
Our understanding of the role of various immune cells and inflammatory factors involved in psoriasis pathogenesis has progressed over the past 20 years. Early clinical studies, such as those with calcineurin inhibitors (Ellis et al, 1986; Griffiths et al, 1986) and agents targeting interleukin-2 (IL-2) receptor–expressing cells (Gottlieb et al, 1995) have demonstrated the integral role of the immune system, and specifically T cells, in psoriasis pathogenesis (Ghoreschi et al, 2007). The central role of proinflammatory factors in the development of psoriasis was demonstrated by the success of therapeutic agents that target tumor necrosis factor (TNF)-α in the treatment of psoriasis (Chaudhari et al, 2001; Leonardi et al, 2003). TNF-α, a proinflammatory factor, is secreted by activated T cells and dendritic cells. The T helper 1 (Th1) subset of activated T cells is the numerically dominant T-cell subset in psoriatic lesions (Kryczek et al, 2008; Lowes et al, 2008) and has been the focus of much attention in psoriasis since the mid-1980s. Many recognized Th1 cytokines and messenger RNAs (mRNAs), including interferon gamma (IFN-γ) and TNF-α, are elevated in psoriatic skin lesions (Austin et al, 1999; Lowes et al, 2008). Development of Th1 cells is driven by IL-12, and a recent therapeutic agent targeting IL-12 through the shared IL-12/23p40 subunit, has also shown strong efficacy (Leonardi et al, 2008). However, more recently, the focus has shifted toward a novel subset of T cells expressing IL-17: Th17 cells, which are also elevated in psoriatic lesions (Lowes et al, 2008; Res et al, 2010). Development of Th17 cells is driven by IL-23 and therefore, like Th1 cells, would be reduced by inhibition of the IL-12/23p40 shared subunit. Results from multiple rodent models of autoimmunity have led to a significant paradigm shift, with Th17 cells and IL-17 replacing the Th1 cells and associated cytokines as dominant mediators of tissue damage (Harrington et al, 2005; Langrish et al, 2005; Park et al, 2005; Steinman, 2007).
Genome-wide association studies and analysis of candidate genomic regions have implicated components of the IL-23 and IL-17 signaling pathways in the development of psoriasis (Table), further focusing attention on Th17 cells in this disease. This review will focus on the emerging role of IL-17 in psoriasis, with particular emphasis on the biology of IL-17 in the skin and the lessons learned from animal models and human clinical studies that confirm IL-17 as a crucial cytokine in psoriasis.
Table 1.
Summary of Genetic Association Data for IL-17–Related Genes Involved in Psoriasis
AS=ankylosing spondylitis; CeD=celiac disease; IBD=inflammatory bowel disease (including Crohn’s disease and ulcerative colitis); IL=interleukin; MS=multiple sclerosis; NF-κB=nuclear factor kappa B; PsA=psoriatic arthritis; RA=rheumatoid arthritis; SLE=systemic lupus erythematosus; SSc=systemic sclerosis
The Role of Interleukin-17 in Immune Defense and in Preclinical Disease Models
T helper 17 cells are CD4+ effector T helper cells that are distinct from the classic Th1 and Th2 lineages. They are defined most notably by the ability to produce large quantities of IL-17A and are thought to have evolved to provide adaptive immunity against pathogens (Gaffen, 2009). Th17 cells differentiate from naive T cells after stimulation with transforming growth factor-β, IL-6, IL-21, and IL-1β (Acosta-Rodriguez et al, 2007; Yang et al, 2008). Th17 cells are subsequently activated by IL-23, IL-1β, and IL-21 to produce multiple inflammatory factors in addition to IL-17A, including IL-17F and IL-22, although this latter cytokine is produced to a much greater extent in murine Th17 cells than in human cells (Awasthi et al, 2009; Ciric et al, 2009; Sutton et al, 2009). In humans, Th17 and a separate type of IL-22–producing cells called Th22 cells have been identified, but the classification of T helper sets is not simple. Human dermal T cells have been shown to produce any combination of IL-17, IL-22, and IFN-γ, although the number of cells uniquely producing either IL-17 or IL-22 exceeds the number producing both (Nograles et al, 2008). The relative production of various cytokines in individual Th17 cells is influenced by the levels of different activating signals, most notably from cytokines and pathogens (Zielinski et al, 2012). In addition to Th17 cells, a subset of cytotoxic T cells (Tc) that also produce IL-17 (Tc17) is present in psoriatic lesions; these cells also produce Th17-related cytokines, including IL-17A (Kryczek et al, 2008), IL-21, and IL-22 (Ortega et al, 2009).
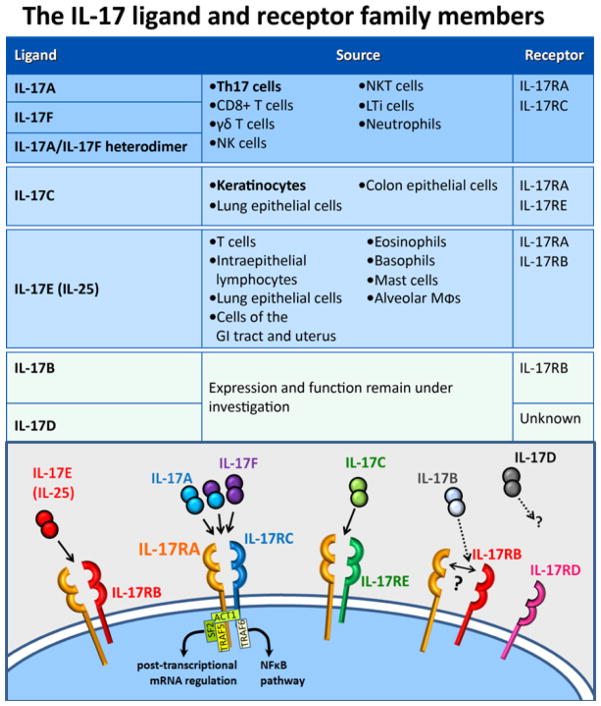
Interleukin-17 is part of a family of cytokines consisting of the prototypical ligand, IL-17 (IL-17A) and 5 other IL-17 ligands (IL-17B through IL-17F; Figure 1) (Gaffen, 2009; Pappu et al, 2010). IL-17A and IL-17F are known to act as homodimers or as IL-17A/F heterodimers (Chang and Dong, 2007; Wright et al, 2007). IL-17E (renamed to IL-25) also exists in a dimeric form (Claudio et al, 2009; Rickel et al, 2008). The IL-17 receptors consist of 5 receptor family members, namely, IL-17 receptor A through IL-17 receptor E. Among the several members of the IL-17 receptor family, IL-17 receptor A contains an extended cytoplasmic domain with unique signaling properties (Gaffen, 2009), and has been implicated as a heteromeric partner for other IL-17 receptors when binding various ligands. IL-17A and IL-17F homodimers and heterodimers signal through the heteromeric receptor consisting of IL-17 receptor A and IL-17 receptor C (Kuestner et al, 2007; Wright et al, 2008). IL-17E (IL-25) signals through a heteromeric receptor containing IL-17 receptor A and IL-17 receptor B (Claudio et al, 2009; Rickel et al, 2008). Recently, IL-17C was demonstrated to require both IL-17 receptor E and IL-17 receptor A for signaling (Ramirez-Carrozzi et al, 2011; Song et al, 2011). Much less is known about IL-17B and IL-17D, but IL-17B is believed to signal through IL-17 receptor B (Gaffen, 2009; Shi et al, 2000). It is unclear whether the receptors for IL-17B and IL-17D are heteromeric, and whether IL-17 receptor A may be a common co-receptor for all IL-17 family ligands.
Figure 1.
IL-17 family ligands and receptors. There are 6 well-defined IL-17 ligands and 5 receptors. The IL-17A, IL-17-F, and IL-17C ligands have elevated expression in psoriatic skin, and have their presumed major cellular sources in the skin highlighted, though other sources may contribute. Those 3 ligands, and IL-17E (IL-25), have demonstrated heteromeric receptor complexes, which in all cases include the IL-17 receptor A subunit, and 1 specific other IL-17 receptor subunit partner. Major cytoplasmic factors interacting with the IL-17 receptor complex are shown, as an example, for the IL-17RA/RC complex, where they are best studied. The IL-17A and IL-17F ligands form homo- and heterodimeric complexes. A dimeric state of the other ligands is illustrated by analogy, but has not been demonstrated, and the exact stoichiometries of the heteromeric receptor complexes are not fully determined. The ligand-receptor interactions are less well defined for IL-17B and IL-17D, and the requirement for an IL-17 receptor A subunit is unknown. IL-17=interleukin-17; IL-17R=IL-17 receptor; NF-κB=nuclear factor kappa B; NK(T)=natural killer (T) cells; Th17=T helper 17;
Interleukin-17A acts on a variety of cell types including endothelial cells, fibroblasts, chondrocytes, synovial cells, monocytes, and epithelial cells including keratinocytes (Harper et al, 2009; Honorati et al, 2001; Yao et al, 1997). IL-17A and IL-17F act directly on keratinocytes to stimulate the production of a number of molecules known to be elevated in psoriasis lesional tissue such as cytokines; β-defensins; antimicrobial peptides (AMPs); and neutrophil-, macrophage-, and lymphocyte-attracting chemokines such as IL-8, CCL20 (also called macrophage inflammatory protein-3 α) and CCL2 (also called monocyte chemotactic protein 1) (Guttman-Yassky et al, 2008). IL-17C, similar to IL-17A and IL-17F, has also recently been demonstrated to act on keratinocytes to induce human β-defensin 2 (hBD2) and granulocyte colony-stimulating factor (Ramirez-Carrozzi et al, 2011). IL-17E (IL-25) is distinct from IL-17A, IL-17C, and IL-17F in that it is generally produced by epithelial cells during an allergic response and acts to induce Th2-type responses (Barlow and McKenzie, 2009; Kleinschek et al, 2007; Rickel et al, 2008). Expression of IL-17E (IL-25), IL-17B, and IL-17D is not increased in lesional psoriatic skin compared with nonlesional skin (Johansen et al, 2009); therefore, these cytokines likely do not play a major role in the development of psoriasis.
There is evidence suggesting involvement of the IL-17 system in antimicrobial defense via maintenance of mucocutaneous immunity. In contrast to the low fungal burdens in mice lacking IL-12 or IL-22, IL-17RA–deficient mice are more susceptible to Candida albicans infections (Huang et al, 2004; Kagami et al, 2010), potentially due to neutrophil and AMP defects (Conti et al, 2011; Gaffen et al, 2011). This susceptibility is also evident in rare human genetic diseases associated with chronic mucocutaneous candidiasis (CMC) that are linked to lack of IL-17 signaling, either through autosomal IL-17RA or IL-17F mutations (Puel et al, 2011) or high titers of neutralizing antibodies to IL-17 cytokines (eg. autoimmune polyendocrine syndrome type 1 due to mutations in the AIRE gene) (Puel et al, 2010). Finally, in a distinct but phenotypically related genetic twist-of-fate, most patients with Hyper-IgE Syndrome (HIES), who tend to be affected by CMC and Staphylococcus aureus infections, have dominant negative mutations in STAT3, which can lead to Th17 and IL-17 deficiency due to a loss in the IL-6 and IL-23 signaling required for Th17 lineage induction and stabilization (Milner et al, 2008). The degree to which these human genetic diseases that affect either IL-17/IL-17R or Th-17 cells (and hence affect a broad number of cytokines) are relevant to partial blockade with antagonists specific to IL-17/IL-17R will be further informed by ongoing clinical studies.
In addition to their potential role in mucocutaneous immunity, Th17 cells also may be involved in vascular inflammation, including atherosclerotic plaque responses to Chlamydophila pneumoniae (Benagiano et al, 2012; Chen et al, 2010). Although atheroma formation in the mouse C. pneumoniae system can be inhibited by genetic defects of IL-17A (Chen et al, 2010), results of biochemical blockade in other murine systems are mixed (Smith et al, 2010; Taleb et al, 2009). A proatherogenic role for IL-17A is supported by the lack of atherosclerotic changes associated with coronary artery defects in patients with HIES (Freeman et al, 2011). Furthermore, patients with psoriasis, who are at increased cardiovascular risk (Mehta et al, 2011), have been reported to have increased circulating IL-17A-producing cells (Bovenschen et al, 2011; Kagami, Rizzo, Lee, et al, 2010).
Expression of Th17-derived cytokines is elevated in murine models of skin inflammation that mimic features of human psoriasis (Blumberg et al, 2010; Nakajima et al, 2011; Singh et al, 2010; van der Fits et al, 2009) and decreases in IL-17A and IL-17F are seen with successful treatments (Blumberg et al, 2010; Singh et al, 2010). Antibodies to IL-17A IL-23p19 or to the shared IL-12/23p40 subunit show efficacy in some models (Blumberg et al, 2010; Nakajima et al, 2011). Data from mouse models have shown that inhibition of IL-23 alone provides comparable efficacy to IL-12/23p40 dual inhibition (Blumberg et al, 2010; Nakajima et al, 2011; Rizzo et al, 2011; Tonel et al, 2010), suggesting that IL-23 is the dominant p40-containing cytokine in psoriasis. IL-23 is known to induce IL-22 in addition to IL-17A, and inhibition of IL-22 has been shown to be efficacious in at least 1 mouse model of psoriasis (Ma et al, 2008). Direct injection of IL-22 into mouse ears or human skin equivalents is sufficient to induce some gene expression changes similar to psoriasis, as well as keratinocyte hyperplasia and parakeratosis (Boniface et al, 2005; Ma et al, 2008; Sa et al, 2007). It is therefore possible that inhibition of IL-22 in addition to IL-17A would be required for full efficacy seen with IL-23 inhibition. However, IL-17 receptor A knockout mice (which lack signaling through multiple IL-17 family members) were shown to be as resistant as IL-23 knockout mice in a psoriasis-like model in which lesions are induced by imiquimod, a toll-like receptor 7/8 ligand (van der Fits et al, 2009). Therefore, we sought to address the question of differential efficacy by comparing IL-23 and IL-17 (both ligand and receptor) inhibition in a preclinical model of skin inflammation (Amgen, unpublished data on file). In this model, IL-36α (IL-36α/IL-1F6; an IL-1 ligand family member) transgenic mice under the control of a keratinocyte (K14) promoter were treated with the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) (Blumberg et al, 2010), leading to the induction of many pathologic features of psoriasis and induction of cytokines and chemokines involved in psoriasis (Blumberg et al, 2010). This model allowed direct comparison of inhibition of IL-12/23p40, IL-23, IL-17A and IL-17F, and IL-17 receptor A. Similar to published studies, IL-12/23p40–and IL-23–specific antagonism was highly efficacious (Blumberg et al, 2010), whereas IL-17A–specific inhibition was only moderately effective (Amgen, unpublished data on file). Antagonism of IL-17F alone was similar to isotype control antibody. Interestingly, inhibition of IL-17 receptor A provided greater benefit over IL-17A specific inhibition and was as efficacious as IL-23 inhibition. These data suggest that combined inhibition of multiple IL-17 family ligands through targeting of IL-17 receptor A may have more profound effects in the skin than inhibition of IL-17A alone and that inhibition of IL-22 is not necessary to achieve efficacy comparable to IL-23 inhibition (Amgen, unpublished data on file). Furthermore, these data suggest that another IL-17 family member in addition to IL-17A and IL-17F may be playing a role in the skin. IL-17C has recently been demonstrated to act on keratinocytes to induce proinflammatory cytokines, chemokines, and AMPs, and this activity requires both IL-17 receptor E and IL-17 receptor A (Ramirez-Carrozzi et al, 2011; Song et al, 2011). Finally, IL-17C knockout mice had significantly decreased inflammation and epidermal thickening in the imiquimod psoriasis-like model (Ramirez-Carrozzi et al, 2011), further suggesting that IL-17C, in addition to IL-17A, could be contributing to the efficacy seen with IL-17 receptor A inhibition in the IL-36 transgenic model of psoriasis. These data collectively suggest that inhibition of 1 or more IL-17 family members has therapeutic potential for psoriasis.
Biology of Interleukin-17 in Human Psoriasis
There is substantial evidence demonstrating the central contribution of Th17 cells and, more directly, IL-17, to plaque psoriasis in humans (Harper et al, 2009; Johansen et al, 2009). Genome-wide association and other genetic studies clearly have linked multiple IL-17–related genes to psoriasis pathogenesis (Table). The molecular and cellular analyses of psoriatic skin have increased our understanding of disease pathogenesis beyond a Th1-driven disease, which was generally accepted until relatively recently. Finally, the remarkable and exciting results in clinical trials have confirmed the central contribution of IL-17 to psoriasis, including both a correlation of IL-17 pathway changes with successful therapy as well as demonstration of the direct effects of inhibition of both IL-17A and IL-17 receptor A.
Expression of IL-17A, IL-17C, and IL-17F is elevated in psoriatic lesional tissue compared with nonlesional tissue (Harper et al, 2009; Johansen et al, 2009; Russell et al, 2011). Paired punch biopsies from lesional skin from patients with psoriasis showed significantly higher protein levels of IL-17A, IL-17C, and IL-17F (6.7-fold, 4.1-fold, and 8-fold higher) compared with nonlesional skin (Johansen et al, 2009).
CD4+ Th17 cells are present in higher numbers in psoriatic lesions than in healthy skin and are decreased after treatment (Lowes et al, 2008). Immunostaining of lesional samples from inflamed skin also showed increased numbers of cells positive for IL-17A (Harper et al, 2009; Lowes et al, 2008). Recent studies have suggested that many of these IL-17–positive cells could be CD8+T cells (Tc17), lymphoid tissue inducer cells, mast cells, and/or neutrophils rather than T helper cells (Cua and Tato, 2010; Lin et al, 2011; Res et al, 2010). The relative contribution of these different cell types will be important in understanding their role in psoriasis pathogenesis.
Human genetic studies have shown that several of the risk alleles involved in psoriasis may be risk alleles for other autoimmune disorders (recently reviewed by Capon et al, 2012 (Capon et al, 2012)) and/or influence IL-17, both upstream of IL-17 expression at the IL-23 cytokine/IL-23R signaling level (Cargill et al, 2007; Nair et al, 2009; Nair et al, 2008) and downstream of the IL-17 receptor (Ellinghaus et al, 2010; Huffmeier et al, 2010). Four of these genes are part of the IL-23/IL-23R signaling system (Table 1) (Cargill et al, 2007; Ellinghaus et al, 2010; Huffmeier et al, 2009; Nair et al, 2009; Strange et al, 2010): IL23A, IL12B, IL23R, and tyrosine kinase 2 (TYK2). IL23A and IL23R are specific to IL-23 signaling and IL12B codes for the shared IL12/23p40 subunit; TYK2 is a signaling kinase downstream of IL-12 and IL-23 (Nakamura et al, 2008). Notably, the association studies have failed to demonstrate a role for IL-12/Th1 pathway–specific genes such as IL12A, IL12RB2, and IFNG. Thus, the genetic evidence aligns more closely with a Th17 rather than Th1 immune driver in psoriasis.
There are a number of genes associated with psoriasis that may function downstream of the IL-17 receptor signaling. Of particular recent interest is TRAF3IP2 coding for the Act-1 protein (also known as TRAF3-interacting protein 2), an intracellular protein which directly binds the IL-17 receptor complex (Ellinghaus et al, 2010). Act-1 can initiate 2 different signal transduction pathways in response to IL-17R activation. NF-κB signaling events can be initiated through interactions with TRAF6 (Schwandner et al, 2000). In the TRAF6-independent pathway, Act-1 can bind to TRAF5 and sequester the RNA binding factor SF2, leading to stabilization of inflammatory mRNAs (Gaffen, 2011; Hartupee et al, 2007; Sun et al, 2011) (Figure 1). The risk haplotypes have a common defect in the ability of Act-1 to bind TRAF6 (Huffmeier et al, 2010) and thus presumably have decreased IL-17R–dependent NF-κB signaling, although this was not specifically demonstrated. This suggests the possibility that the psoriasis risk may be driven by increased proinflammatory activity through the Act1-TRAF5- mRNA stabilization pathway. Additional candidate genes implicated in psoriasis include other NF-κB pathway regulatory genes (REL, TNIP1, TNFAIP3, and NFKBIA) and increased copy number of the gene segment harboring the IL-17–regulated gene DEFB4, which encodes for hBD2 (Table 1).
The presence of both Th1 and Th17 subsets in psoriasis may seem counterintuitive given that these 2 polarized subsets have counterinhibitory effects on each other (Bettelli et al, 2006). Under certain conditions, however, such as those that exist in psoriatic lesions, Th1 cells promote expansion of Th17 cells (Kryczek et al, 2008). In addition, there appears to be considerable plasticity in this system; Th17 cells have the ability to differentiate into IFN-γ–producing Th1 cells (Hirota et al, 2011; Nistala et al, 2010) although not vice versa. CD8+ T cells are found in increased numbers in the psoriatic epidermis, including subsets producing IFN-γ (Tc1), IL-17 (Tc17), and/or IL-22 (Tc22) (Res et al, 2010). Similar to Th17 cells, Tc17 cells are dependent on IL-23 for maintenance and expansion (Ciric et al, 2009). The notable presence of Tc17 cells in the epidermis, where IL-17 receptor is abundantly expressed on keratinocytes, suggests a potential pathogenic role for this subset of IL-17-producing cells (Figure 2).
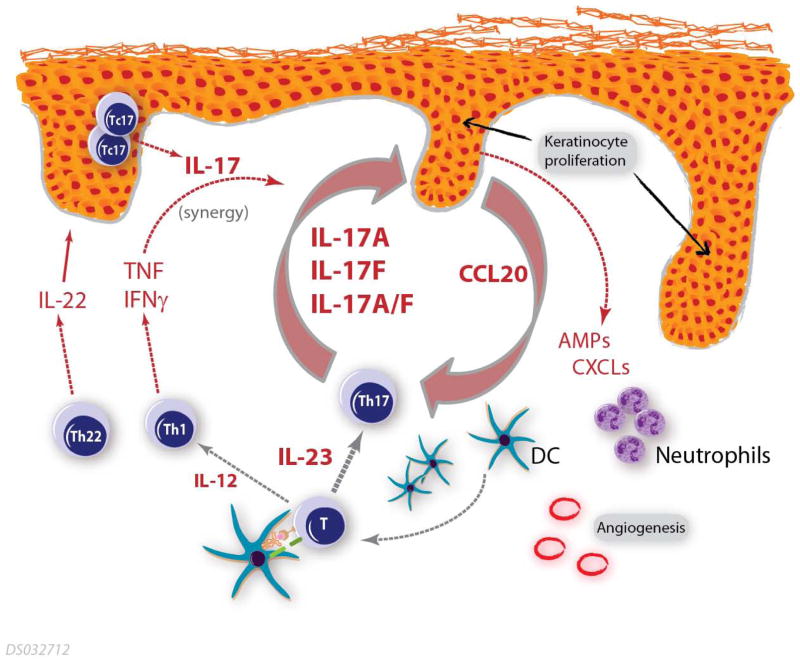
Figure 2.
A model for the central role of IL-17 in psoriasis pathogenesis. This model includes core inflammatory elements that establish a self-reinforcing cycle, including Th17 skewing of naive T cells in the presence of IL-23 leading to the local production of IL-17 ligands. Keratinocytes in turn are stimulated by these IL-17 ligands leading to an aberrant differentiation program and elevated production of proinflammatory factors including AMPs and chemokines (including CCL20, which attracts both Th17 cells and DCs). These keratinocyte-derived factors in turn stimulate further recruitment of inflammatory cells, including IL-17 producing cells, and establish a self-sustaining inflammatory feedback loop. AMPs=antimicrobial peptides; DCs=dendritic cells; IL-12=interleukin-12; IL-17=interleukin-17; IL-23=interleukin-23; Th1=T helper 1; Th17=T helper 17; Th22=T helper 22.
Global gene expression studies have consistently demonstrated the upregulation of the IL-17 pathway in psoriasis. Several studies have used quantitative real-time polymerase chain reaction assays to show increased expression of IL-17 ligands and the IL-23 subunits in lesional skin compared with both nonlesional and normal skin (Harper et al, 2009; Johansen et al, 2009; Lowes et al, 2008; Zaba et al, 2007). Microarray studies demonstrate that many thousands of transcripts are differentially regulated in the disease state (Gudjonsson et al, 2010; Yao et al, 2008), and successful therapy is accompanied by rapid and nearly complete reversion of expression of a wide range of genes (including cytokines, AMPs, and downstream effector molecules) to near normal (nonlesional) levels, suggesting that these alterations in gene expression are necessary for therapeutic response (Russell et al, 2011; Russell et al, 2010; Zaba et al, 2009). Again, elements upstream and downstream of IL-17 show higher expression, implicating the pathway in psoriasis. Notably, among the genes that are universally overexpressed across several studies are those for IL-17A and IL-17F; IL-23; and a number of proinflammatory genes regulated by IL-17A in keratinocytes, such as β-defensin, neutrophil chemoattractants, and the chemokine CCL20 (Nograles et al, 2008).
Consistent with the results described above is the observation that IL-17 ligands (including both IL-17A and IL-17C) and TNF-α amplify each other’s effects, both additively and synergistically, and their combined activity accounts for many of the key inflammatory pathways in psoriasis (Chiricozzi et al, 2011). Some of this synergy may be the result of the distinct mechanisms by which IL-17 and TNF each regulate downstream gene expression. The TNF receptor stimulates several pathways that activate gene transcription while major activities downstream of the IL-17 receptor include modulation of NF-κB signaling and stabilization of mRNAs induced by TNF (Hartupee et al, 2007; Sun et al, 2011). The synergistic interaction between IL-17 and TNF, as well as the regulation of IL-17 by IL-23, is a potential unifying theme in understanding the success of different therapeutic agents in psoriasis, including the calcineurin inhibitors, and antibodies targeting TNF, IL-12/23-p40, IL-23p19, IL-17A, and IL-17 receptor A.
Role of Interleukin-17 Inhibition in the Clinic
Along with our understanding of disease pathophysiology, therapeutic efficacy in psoriasis has also advanced tremendously over the past 20 years. Clinical response to TNF antagonism in psoriasis has been shown to correlate with the rapid early reduction of IL-23 and IL-17A, followed by later reductions in Th1-associated genes (Zaba et al, 2007). Studies with IL-12/23p40 (Leonardi et al, 2008; Papp et al, 2008) and IL-23p19 (Sofen et al, 2011) antagonists have also demonstrated clinical efficacy and support the role of IL-12/IL-23 and Th17 cells in the pathogenesis of psoriasis. Although the key cytokines released from human Th17 cells that drive psoriasis pathogenesis, including IL-22 and/or IL-17, remain unclear, direct antagonism of the IL-17A ligand (Genovese et al, 2010; Hueber et al, 2010; Hueber et al, 2011) and the IL-17 receptor with brodalumab (Papp et al, 2012; Russell et al, 2011; Russell et al, 2010) have demonstrated striking results in early-phase clinical trials in psoriasis.
Secukinumab (AIN457) is an anti–IL-17A ligand monoclonal antibody that has been assessed for the treatment of psoriasis, rheumatoid arthritis (RA), chronic noninfectious uveitis, and Crohn’s disease (Hueber et al, 2010; Hueber et al, 2011). A phase 1 study in 36 patients with psoriasis showed that a single dose of secukinumab at 3 mg/kg significantly reduced disease severity (mean psoriasis area and severity index [PASI] score) by 63% at week 12 versus 9% for placebo (Hueber et al, 2010). Clinical responses were associated with reductions in epidermal hyperplasia, expression of IL-17A+ and CD3+ cells from immunostained micrographs, and gene expression of various cytokines and chemokines (eg, IL-17A, IL-21, IL-22, CCL20, KRT16, and DEFB4) (Hueber et al, 2010). Data from 3 phase 2 studies showed 12-week PASI75 response rates of 81% in patients with psoriasis treated with subcutaneous secukinumab 150 mg (3 or 4 doses) and of 83% in patients treated with intravenous secukinumab 10 mg/kg (3 doses) (Papp, 2011a; Papp, 2011b; Rich, 2011). LY2439821 (ixekinumab) is a monoclonal antibody to IL-17A that is being investigated for the treatment of psoriasis and RA (Genovese et al, 2010). Recent data from a phase 2 study showed week 12 PASI75 response rates of 77%, 83%, and 82% following administration of subcutaneous ixekinumab 25 mg, 75 mg, and 150 mg (weeks 0, 2, 4, 8, and 12), respectively, versus 7.7% for placebo (Leonardi et al, 2012). Up to 72% of patients in the active-treatment groups achieved clear/almost clear status according to Physician’s Global Assessment.
Brodalumab (AMG 827) is an IL-17 receptor A monoclonal antibody that is under investigation for a number of inflammatory conditions, including psoriasis, psoriatic arthritis, and asthma. A phase 1 proof-of-concept study in patients with psoriasis demonstrated significant improvements in clinical parameters: 7 of 8 and 5 of 8 patients achieved PASI75 by week 6 in the high (700 mg intravenous) and moderate (350 mg subcutaneous) single-dose groups, respectively (Russell et al, 2011; Russell et al, 2010). Improvements in histopathological parameters included reductions in epidermal thickness, Ki-67 counts (an index of proliferation-specific nuclear antigen) (Gerdes et al, 1983), keratin-16 gene expression, and infiltrating leukocyte subsets (Russell et al, 2011; Russell et al, 2010). In a phase 2 dose-ranging study, AMG 827 (70, 140, or 210 mg at weeks 0, 1, and 2, then every other week or 280 mg monthly) produced dose-dependent improvements in PASI and Physician Global Assessment responses (Papp et al, 2012). Significant mean PASI improvements at week 12 were 45%, 86%, 86%, and 76% in the AMG 827 70-, 140-, 210-, and 280-mg groups, compared with 16% in the placebo group. PASI75 responses were observed in 33%, 77%, 82%, and 67% of patients, respectively, in the AMG 827 groups and 0% in the placebo group. Similar results were observed for other PASI measures and Physician’s Global Assessment, with up to 85% of patients in the AMG 827 groups achieving clear/almost clear status.
The significant clinical responses in patients with psoriasis following blockade of either IL-17 receptor A (with brodalumab) or its ligand IL-17A (with secukinumab and ixekinumab) confirms the hypothesis that IL-17 signaling plays a critical role in this immune-mediated disease. Furthermore, the complete reversal of regenerative epidermal hyperplasia and high PASI responses seen with AMG 827 implies that factors signaling through the IL-17 receptor A, including IL-17A, IL-17C and IL-17F, are central drivers of psoriasis immunopathogenesis.
An Emerging Model for the Role of Interleukin-17 in Psoriasis Pathogenesis
This review has focused on the emerging role of IL-17 in the pathogenesis of psoriasis, including preclinical results, genetic data and both indirect and direct evidence from clinical studies. These findings demonstrate the central importance of this innate family of proinflammatory cytokines in psoriasis. A model for the role of various factors in psoriasis pathogenesis is presented in Figure 2. Notable features in this model include a set of core inflammatory elements that establish a self-reinforcing cycle, along with ancillary proinflammatory elements that synergize with and amplify the core elements. The core elements include: (a) naive T-cell skewing into the Th17 lineage following interaction with activated dendritic cells (DCs) in the presence of IL-23; (b) local Th17 production of IL-17A and IL-17F; and (c) keratinocyte stimulation by IL-17 ligands leading to aberrant differentiation and proliferation and the production of proinflammatory AMPs, chemokines, and angiogenic factors, which in turn stimulate further recruitment of inflammatory cells, setting up a positive feedback loop. The chemokine CCL20 is a notable keratinocyte-derived core component that functions to recruit both inflammatory DCs and Th17 cells into active lesional skin. Additional ancillary factors in this inflammatory network include cytokines (eg, IL-22, which promotes keratinocyte alterations, and TNF, which can synergize with IL-17 ligands to promote inflammation); Th1 and Th22 cells, which amplify the core response via the production of inflammatory factors; and keratinocyte-derived angiogenic and chemoattractant factors. The core and ancillary elements in this model function together to establish an activated inflammatory network, ultimately resulting in the formation of psoriasis lesions.
Advances in genetics and immunology have enabled the development of targeted therapeutics in psoriasis, a number of which have shown impressive clinical efficacy. Clinical and molecular data from these therapeutic successes have provided additional opportunities to focus on and identify inhibitors or central drivers of psoriasis pathogenesis, including therapeutics targeting the IL-17 family. As our understanding of psoriasis pathogenesis increases, the parallel evolution of increasingly selective therapies may provide patients with an optimal balance between increased clinical benefit and reduced risk for side effects. The early clinical results from inhibition of IL-17 (ligand or receptor) have established this cytokine as a core downstream element in psoriasis, and ongoing late-phase studies will allow further confirmation of these findings.
Acknowledgments
The authors wish to thank Rick Davis, MS, RPh, whose work was funded by Amgen Inc., and Meera Kodukulla, PhD, of Amgen Inc., for assistance in drafting this manuscript. J. Gudjonsson is supported by NIH K08 AR060802 and A. Alfred Taubman Medical Research Institute as the Kenneth and Frances Eisenberg Emerging Scholar. The authors also thank Dirk Smith, of Amgen Inc., for graphical support.
Footnotes
Conflict of interest statement:
D. Martin, C. Russell, J. Towne, G. Kricorian, and P. Klekotka are employees and stock holders of Amgen. J. Gudjonsson has been a consultant for Novartis. J. Krueger has been a consultant for Astellas, Amgen, Biogen, Boehringer, Centocor (Janssen), Celgene, GSK, Lilly, Merck, Novartis, and Pfizer; has been an investigator for Boehringer, Centocor (Janssen), Lilly, Merck, Novartis, and Pfizer; has received honoraria from Astellas, Biogen, Boehringer, Centocor (Janssen), Celgene, Lilly, Merck, and Pfizer; and has received research grants from Amgen, Boehringer, Centocor (Janssen), Lilly, Merck, and Pfizer.
Contributor Information
Dr. David A. Martin, Medical Sciences, Amgen Inc., Seattle, WA, USA.
Dr. Jennifer E. Towne, Inflammation Research, Amgen Inc., Seattle, WA, USA.
Dr. Gregory Kricorian, Clinical Development, Amgen Inc., Thousand Oaks, CA, USA.
Dr. Paul Klekotka, Clinical Development, Amgen Inc., Thousand Oaks, CA, USA.
Dr. Johann E. Gudjonsson, Department of Dermatology, University of Michigan, Ann Arbor, MI, USA.
Dr. James G. Krueger, Laboratory of Investigative Dermatology, Rockefeller University, New York, NY, USA.
Dr. Chris B. Russell, Medical Sciences, Amgen Inc., Seattle, WA, USA.
References
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Allanore Y, Saad M, Dieude P, Avouac J, Distler JH, Amouyel P, et al. Genome-wide scan identifies TNIP1, PSORS1C1, and RHOB as novel risk loci for systemic sclerosis. PLoS Genet. 2011;7:e1002091. doi: 10.1371/journal.pgen.1002091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–759. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- Australia and New Zealand Multiple Sclerosis Genetics Consortium. Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet. 2009;41:824–828. doi: 10.1038/ng.396. [DOI] [PubMed] [Google Scholar]
- Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban M, Goris A, Lorentzen AR, Baker A, Mihalova T, Ingram G, et al. Replication analysis identifies TYK2 as a multiple sclerosis susceptibility factor. Eur J Hum Genet. 2009;17:1309–1313. doi: 10.1038/ejhg.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow JL, McKenzie AN. IL-25: a key requirement for the regulation of type-2 immunity. Biofactors. 2009;35:178–182. doi: 10.1002/biof.24. [DOI] [PubMed] [Google Scholar]
- Benagiano M, Munari F, Ciervo A, Amedei A, Paccani SR, Mancini F, et al. Chlamydophila pneumoniae phospholipase D (CpPLD) drives Th17 inflammation in human atherosclerosis. Proc Natl Acad Sci U S A. 2012;109:1222–1227. doi: 10.1073/pnas.1111833109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Dinh H, Dean C, Jr, Trueblood ES, Bailey K, Shows D, et al. IL-1RL2 and its ligands contribute to the cytokine network in psoriasis. J Immunol. 2010;185:4354–4362. doi: 10.4049/jimmunol.1000313. [DOI] [PubMed] [Google Scholar]
- Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol. 2011;131:1853–1860. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- Capon F, Burden AD, Trembath RC, Barker JN. Psoriasis and other complex trait dermatoses: from Loci to functional pathways. J Invest Dermatol. 2012;132:915–922. doi: 10.1038/jid.2011.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357:1842–1847. doi: 10.1016/s0140-6736(00)04954-0. [DOI] [PubMed] [Google Scholar]
- Chen S, Shimada K, Zhang W, Huang G, Crother TR, Arditi M. IL-17A is proatherogenic in high-fat diet-induced and Chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J Immunol. 2010;185:5619–5627. doi: 10.4049/jimmunol.1001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, Cardinale I, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677–687. doi: 10.1038/jid.2010.340. [DOI] [PubMed] [Google Scholar]
- Ciric B, El-behi M, Cabrera R, Zhang GX, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol. 2009;182:5296–5305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- Claudio E, Sonder SU, Saret S, Carvalho G, Ramalingam TR, Wynn TA, et al. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J Immunol. 2009;182:1617–1630. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, et al. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 2011;4:448–455. doi: 10.1038/mi.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus E, Ellinghaus D, Stuart PE, Nair RP, Debrus S, Raelson JV, et al. Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat Genet. 2010;42:991–995. doi: 10.1038/ng.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus E, Stuart PE, Ellinghaus D, Nair RP, Debrus S, Raelson JV, et al. Genome-Wide Meta-Analysis of Psoriatic Arthritis Identifies Susceptibility Locus at REL. J Invest Dermatol. 2012;132:1133–1140. doi: 10.1038/jid.2011.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CN, Gorsulowsky DC, Hamilton TA, Billings JK, Brown MD, Headington JT, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110–3116. [PubMed] [Google Scholar]
- Filer C, Ho P, Smith RL, Griffiths C, Young HS, Worthington J, et al. Investigation of association of the IL12B and IL23R genes with psoriatic arthritis. Arthritis Rheum. 2008;58:3705–3709. doi: 10.1002/art.24128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SA, Tremelling M, Anderson CA, Gwilliam R, Bumpstead S, Prescott NJ, et al. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn’s disease. Nat Genet. 2008;40:710–712. doi: 10.1038/ng.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AF, Avila EM, Shaw PA, Davis J, Hsu AP, Welch P, et al. Coronary artery abnormalities in Hyper-IgE syndrome. J Clin Immunol. 2011;31:338–345. doi: 10.1007/s10875-011-9515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opin Immunol. 2011;23:613–619. doi: 10.1016/j.coi.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL, Hernandez-Santos N, Peterson AC. IL-17 signaling in host defense against Candida albicans. Immunol Res. 2011;50:181–187. doi: 10.1007/s12026-011-8226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. 2009;41:1228–1233. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K, Weigert C, Rocken M. Immunopathogenesis and role of T cells in psoriasis. Clin Dermatol. 2007;25:574–580. doi: 10.1016/j.clindermatol.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Gottlieb SL, Gilleaudeau P, Johnson R, Estes L, Woodworth TG, Gottlieb AB, et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med. 1995;1:442–447. doi: 10.1038/nm0595-442. [DOI] [PubMed] [Google Scholar]
- Graham RR, Cotsapas C, Davies L, Hackett R, Lessard CJ, Leon JM, et al. Genetic variants near TNFAIP3 on 6q23 are associated with systemic lupus erythematosus. Nat Genet. 2008;40:1059–1061. doi: 10.1038/ng.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen PK, Amos CI, Lee AT, Lu Y, Remmers EF, Kastner DL, et al. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat Genet. 2009;41:820–823. doi: 10.1038/ng.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths CE, Powles AV, Leonard JN, Fry L, Baker BS, Valdimarsson H. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed) 1986;293:731–732. doi: 10.1136/bmj.293.6549.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Ding J, Johnston A, Tejasvi T, Guzman AM, Nair RP, et al. Assessment of the psoriatic transcriptome in a large sample: additional regulated genes and comparisons with in vitro models. J Invest Dermatol. 2010;130:1829–1840. doi: 10.1038/jid.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535–546. doi: 10.1016/j.clindermatol.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181:7420–7427. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175–2183. doi: 10.1038/jid.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40:23–25. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honorati MC, Meliconi R, Pulsatelli L, Cane S, Frizziero L, Facchini A. High in vivo expression of interleukin-17 receptor in synovial endothelial cells and chondrocytes from arthritis patients. Rheumatology. 2001;40:522–527. doi: 10.1093/rheumatology/40.5.522. [DOI] [PubMed] [Google Scholar]
- Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- Hueber W, Sands B, Vandemeulebroecke M, Reinisch W, Higgins P, Wehkamp J, et al. Inhibition of IL-17A by secukinumab is ineffective for Crohn’s disease (CD) [abstract 10]. Paper presented at the 6th Congress of the European Crohn’s and Colitis Organisation-Inflammatory Bowel Diseases; Dublin, Ireland. 2011. Feb 24–26, [Google Scholar]
- Huffmeier U, Lascorz J, Bohm B, Lohmann J, Wendler J, Mossner R, et al. Genetic variants of the IL-23R pathway: association with psoriatic arthritis and psoriasis vulgaris, but no specific risk factor for arthritis. Journal of Investigative Dermatology. 2009;129:355–358. doi: 10.1038/jid.2008.233. [DOI] [PubMed] [Google Scholar]
- Huffmeier U, Uebe S, Ekici AB, Bowes J, Giardina E, Korendowych E, et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat Genet. 2010;42:996–999. doi: 10.1038/ng.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Yang P, Hou S, Du L, Xie L, Zhou H, et al. IL-23R gene confers susceptibility to Behcet’s disease in a Chinese Han population. Ann Rheum Dis. 2010;69:1325–1328. doi: 10.1136/ard.2009.119420. [DOI] [PubMed] [Google Scholar]
- Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319–324. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. 2010;185:5453–5462. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast Cells and Neutrophils Release IL-17 through Extracellular Trap Formation in Psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta NN, Yu Y, Pinnelas R, Krishnamoorthy P, Shin DB, Troxel AB, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124:775 e771–776. doi: 10.1016/j.amjmed.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuki N, Meguro A, Ota M, Ohno S, Shiota T, Kawagoe T, et al. Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behcet’s disease susceptibility loci. Nat Genet. 2010;42:703–706. doi: 10.1038/ng.624. [DOI] [PubMed] [Google Scholar]
- Musone SL, Taylor KE, Lu TT, Nititham J, Ferreira RC, Ortmann W, et al. Multiple polymorphisms in the TNFAIP3 region are independently associated with systemic lupus erythematosus. Nat Genet. 2008;40:1062–1064. doi: 10.1038/ng.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair RP, Ruether A, Stuart PE, Jenisch S, Tejasvi T, Hiremagalore R, et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol. 2008;128:1653–1661. doi: 10.1038/sj.jid.5701255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Kanda T, Takaishi M, Shiga T, Miyoshi K, Nakajima H, et al. Distinct roles of IL-23 and IL-17 in the development of psoriasis-like lesions in a mouse model. J Immunol. 2011;186:4481–4489. doi: 10.4049/jimmunol.1000148. [DOI] [PubMed] [Google Scholar]
- Nakamura R, Shibata K, Yamada H, Shimoda K, Nakayama K, Yoshikai Y. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by gammadelta T cells. J Immunol. 2008;181:2071–2075. doi: 10.4049/jimmunol.181.3.2071. [DOI] [PubMed] [Google Scholar]
- Naldi L. Epidemiology of psoriasis. Curr Drug Targets Inflamm Allergy. 2004;3:121–128. doi: 10.2174/1568010043343958. [DOI] [PubMed] [Google Scholar]
- Nistala K, Adams S, Cambrook H, Ursu S, Olivito B, de Jager W, et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci U S A. 2010;107:14751–14756. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega C, Fernandez AS, Carrillo JM, Romero P, Molina IJ, Moreno JC, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol. 2009;86:435–443. doi: 10.1189/JLB.0109046. [DOI] [PubMed] [Google Scholar]
- Papp KA. Secukinumab efficacy and safety preliminary results from a phase II subcutaneous dose-ranging study in the treatment of moderate-to-severe plaque psoriasis [oral presentation FC01.5]. Paper presented at the 20th Congress of the European Academy of Dermatology and Venereology; Lisbon, Portugal.. 2011a. Oct 20–24, [Google Scholar]
- Papp KA. Secukinumab, a noval fully human antibody to interleukin-17A, in the treatment of moderate-to-severe plaque psoriasis: efficacy and safety interim results from a phase II intravenous induction dose-ranging study [oral presentation FC01.7]. Paper presented at the 20th Congress of the European Academy of Dermatology and Venereology..2011b. Oct 20–24, [Google Scholar]
- Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- Pappu R, Ramirez-Carrozzi V, Ota N, Ouyang W, Hu Y. The IL-17 family cytokines in immunity and disease. J Clin Immunol. 2010;30:185–195. doi: 10.1007/s10875-010-9369-6. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsopoulos, NA, Bayer Pharma, MSGWG, Steering Committees of Studies Evaluating, I-b, a, CCRA, Consortium, AN, GeneMsa, et al. Genome-wide meta-analysis identifies novel multiple sclerosis susceptibility loci. Ann Neurol. 2011;70:897–912. doi: 10.1002/ana.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenge RM, Cotsapas C, Davies L, Price AL, de Bakker PI, Maller J, et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat Genet. 2007;39:1477–1482. doi: 10.1038/ng.2007.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12:1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- Remmers EF, Cosan F, Kirino Y, Ombrello MJ, Abaci N, Satorius C, et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behcet’s disease. Nat Genet. 2010;42:698–702. doi: 10.1038/ng.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Res PC, Piskin G, de Boer OJ, van der Loos CM, Teeling P, Bos JD, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS ONE. 2010;5:e14108. doi: 10.1371/journal.pone.0014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich PA. Secukinumab, a new fully human monoclonal anti-interleukin-17A antibody, in the treatment of moderate-to-severe plaque psoriasis: Interim efficacy and safety data from a phase II regimen-finding trial [oral presentation FC01.6]. Paper presented at the 20th Congress of the European Academy of Dermatology and Venereology; Lisbon, Portugal.. 2011. Octoboer. [Google Scholar]
- Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, Blauvelt A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol. 2011;186:1495–1502. doi: 10.4049/jimmunol.1001001. [DOI] [PubMed] [Google Scholar]
- Russell C, Kerkof K, Bigler J, Timour M, Welcher A, Bautista E, et al. Blockade of the IL-17R with AMG 827 leads to rapid reversal of gene expression and histopathologic abnormalities in psoriatic skin, including substantial pathway-specific effects within one week [abstract 065] J Invest Dermatol. 2011;131:S11. [Google Scholar]
- Russell C, Kerkof K, Bigler J, Timour M, Welcher A, Novitskaya I, et al. Blockade of the IL-17R with AMG 827 leads to rapid reversal of gene expression and histopathologic abnormalities in human psoriatic skin [abstract 273] J Invest Dermatol. 2010;130:S46. [Google Scholar]
- Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Ullrich SJ, Zhang J, Connolly K, Grzegorzewski KJ, Barber MC, et al. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J Biol Chem. 2000;275:19167–19176. doi: 10.1074/jbc.M910228199. [DOI] [PubMed] [Google Scholar]
- Sigurdsson S, Nordmark G, Goring HH, Lindroos K, Wiman AC, Sturfelt G, et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet. 2005;76:528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh TP, Schon MP, Wallbrecht K, Michaelis K, Rinner B, Mayer G, et al. 8-methoxypsoralen plus ultraviolet A therapy acts via inhibition of the IL-23/Th17 axis and induction of Foxp3+ regulatory T cells involving CTLA4 signaling in a psoriasis-like skin disorder. J Immunol. 2010;184:7257–7267. doi: 10.4049/jimmunol.0903719. [DOI] [PubMed] [Google Scholar]
- Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofen H, Smith S, Matheson R, Leonardi C, Calderon C, Bouman-Thio E, et al. Results of a single ascending dose study to assess the safety and tolerability of CNTO 1959 following intravenous or subcutaneous administration in healthy subjects and in subjects with moderate to severe psoriasis [abstract FC-21] Br J Dermatol. 2011;165:E10. [Google Scholar]
- Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin SD, et al. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol. 2011;12:1151–1158. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Strange A, Capon F, Spencer CC, Knight J, Weale ME, Allen MH, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985–990. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart PE, Nair RP, Ellinghaus E, Ding J, Tejasvi T, Gudjonsson JE, et al. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat Genet. 2010;42:1000–1004. doi: 10.1038/ng.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF) Nat Immunol. 2011;12:853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson W, Barton A, Ke X, Eyre S, Hinks A, Bowes J, et al. Rheumatoid arthritis association at 6q23. Nat Genet. 2007;39:1431–1433. doi: 10.1038/ng.2007.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonel G, Conrad C, Laggner U, Di Meglio P, Grys K, McClanahan TK, et al. Cutting edge: A critical functional role for IL-23 in psoriasis. J Immunol. 2010;185:5688–5691. doi: 10.4049/jimmunol.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trynka G, Zhernakova A, Romanos J, Franke L, Hunt KA, Turner G, et al. Coeliac disease-associated risk variants in TNFAIP3 and REL implicate altered NF-kappaB signalling. Gut. 2009;58:1078–1083. doi: 10.1136/gut.2008.169052. [DOI] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- Burton PR, Clayton DG, Cardon LR, Craddock N, et al. Wellcome Trust Case Control C, Australo-Anglo-American Spondylitis C. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JF, Bennett F, Li B, Brooks J, Luxenberg DP, Whitters MJ, et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol. 2008;181:2799–2805. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem. 2007;282:13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Richman L, Morehouse C, de los Reyes M, Higgs BW, Boutrin A, et al. Type I interferon: potential therapeutic target for psoriasis? PLoS ONE. 2008;3:e2737. doi: 10.1371/journal.pone.0002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Spriggs MK, Derry JM, Strockbine L, Park LS, VandenBos T, et al. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine. 1997;9:794–800. doi: 10.1006/cyto.1997.0240. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez-Farinas M, Fuentes-Duculan J, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Suarez-Farinas M, Fuentes-Duculan J, Nograles KE, Guttman-Yassky E, Cardinale I, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124:1022–1010. e1021–1395. doi: 10.1016/j.jaci.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XJ, Huang W, Yang S, Sun LD, Zhang FY, Zhu QX, et al. Psoriasis genome-wide association study identifies susceptibility variants within LCE gene cluster at 1q21. Nat Genet. 2009;41:205–210. doi: 10.1038/ng.310. [DOI] [PubMed] [Google Scholar]
- Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, et al. Pathogen-induced human T(H)17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012 doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]