Figure 3. HF interacts with both the site for amino acid activation and the site for docking the 3′-end of tRNA.
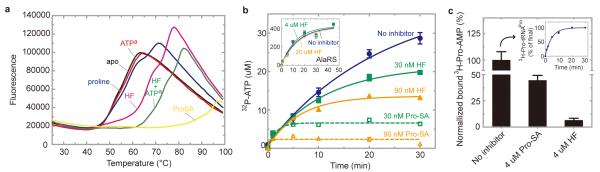
a, Thermal melting19 of ProRS in the presence of different ligands. HF binds in the absence of ATPa, and binds more strongly in the presence of ATPa. b, HF and the comparator Pro-SA block formation of Pro-AMP, in the proline-dependent ATP-PPi exchange reaction. The inset shows that HF had no effect on the alanine-dependent ATP-PPi exchange reaction with AlaRS. c, HF mobilizes the release of Pro-AMP from ProRS. The ProRS:3H-Pro-AMP complex was prepared on ice and then isolated on a column and for 10 min was left untreated, or exposed to HF, or exposed to Pro-SA, respectively. The complex was then re-run on the column and the amount of bound 3H-Pro-AMP was determined. The inset shows that the isolated 3H-Pro was activated (as 3H-Pro-AMP) and could be transferred to tRNA. Error bars are s.e.m. (n=2).
