Table 1.
Focused Pi group heterocyclic library (53-61).
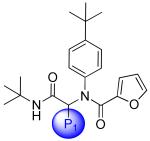
|
|||
|---|---|---|---|
|
| |||
| Compound | SID |
|
3CLpro IC50μM |
| 53 | 117686897 |
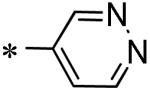
|
10 |
| 54 | 117686898 |
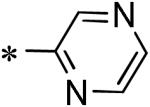
|
5.5 |
| 55 | 117686909 |
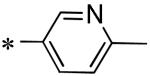
|
Inactive |
| 56 | 117686901 |
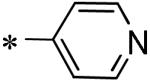
|
45 |
| 57 | 117686900 |
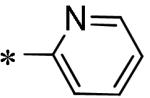
|
Inactive |
| 58 | 117686899 |
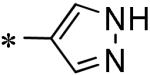
|
16% (100 μM) |
| 59 | 117686904 |
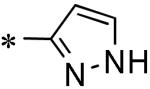
|
Inactive |
| 60 | 117686905 |
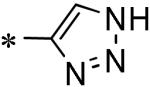
|
23% (100 μM) |
| 61 | 117686906 |
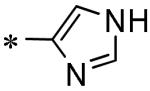
|
23% (100 μM) |
IC50values are average of two independent runs using triplicate concentrations, ‘Inactive’ defined as %inhibition <15% at 100 μM, CV <0.3
