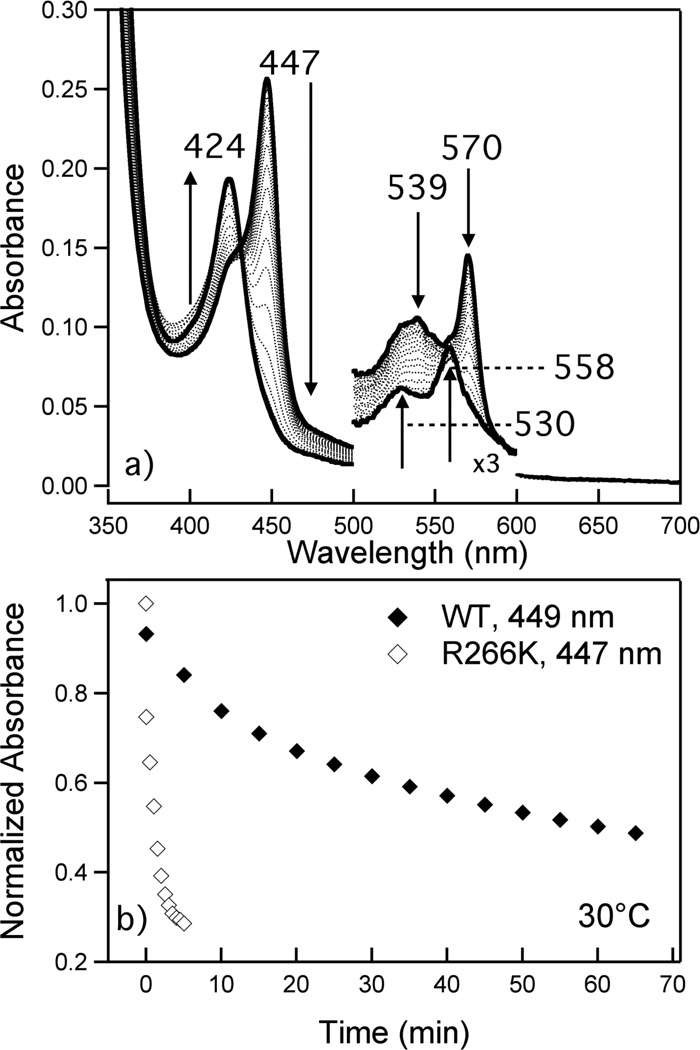
Figure 7.
Ligand switch process of Fe(II) R266K hCBS at 30°C. Protein (3.8 µM) was in 100 mM CHES buffer, 100 mM NaCl, pH 8.6; sodium dithionite was added to a final concentration of 1.5 mM. (A) Solid lines indicate the initial (447 nm Soret) and final (424 nm Soret) spectra; dotted spectra were taken at 1 min intervals after addition of reductant. (B) Time course plots showing the loss of the Fe(II) WT hCBS Cys(thiolate)-ligated heme Soret at 449 nm (♦) and the loss of the Fe(II) R266K hCBS Cys(thiolate)-ligated heme Soret at 447 nm (◊).