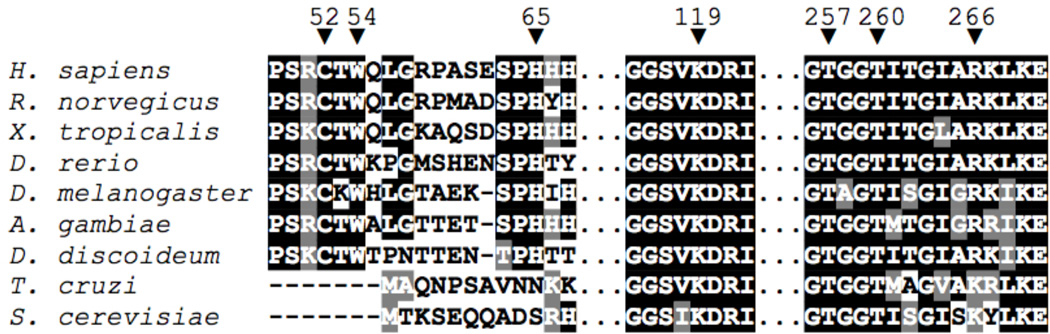
Figure 8.
Partial sequence alignment of CBS enzymes from selected species, with human enzyme numbering. Heme ligands Cys52 and His65, PLP-internal-aldimine forming Lys119, Cys52(thiolate)-contacts Trp54 and Arg266, and PLP-contacts Thr257 and Thr260 are all labeled with an inverted arrow (▼). Human (H. sapiens accession no. P35520), rat (R. norvegicus, P32232), frog (X. tropicalis, Q640V0), zebrafish (D. rerio, B7ZV69), fruit fly (D. melanogaster, Q9VRD9), mosquito (A. gambiae, Q7QEV0) and slime mold (D. discoideum, P46794) sequences all contain the necessary ligands for heme binding and have Arg266 completely conserved. Trypanosoma (T. cruzi, Q9BH24) and yeast (S. cerevisiae, P32582) CBS, which do not contain heme or its associated ligands, have a Lys residue at the position that is analogous to human Arg266. Sequence alignments were visualized using the MEGA5 program (70).