Abstract
The purpose of the study was to compare the discharge characteristics of biceps brachii motor units of young and old adults when they performed steady, submaximal contractions while the arm supported different inertial loads. Young (28 ± 4 yr; n = 16) and old (75 ± 4 yr; n = 14) adults performed steady contractions with the elbow flexors at target forces set at either small (11.7 ± 4.4% maximum) or large (17.8 ± 6.5% maximum) differences below the recruitment threshold force of the motor unit (n = 40). The task was to maintain an elbow angle at 1.57 rad until the motor unit was recruited and discharged action potentials for ∼120 s. Time to recruitment was longer for the larger target force difference (187 ± 227 s vs. 23 ± 46 s, P < 0.001). Once recruited, motor units discharged action potentials either repetitively or intermittently, with a greater proportion of motor units exhibiting the repetitive pattern for old adults. Discharge rate at recruitment and during the steady contraction was similar for the two target force differences for old adults but was greater for the small target force difference for young adults. Discharge variability was similar at recruitment for the two age groups but less for the old adults during the steady contraction. The greatest difference between the present results and those reported previously when the arm pulled against a rigid restraint was that old adults modulated discharge rate less than young adults across the two contraction intensities for both load types.
Keywords: aging, biceps brachii, load type, motor unit recruitment, discharge rate modulation
the decline in motor performance with advancing age appears to be precipitated by adaptations in the activation of muscle by the central nervous system rather than by decreases in skeletal muscle mass (Clark and Manini 2008; Delbono 2003; Roos et al. 1997; Vandervoort 2002). Time to complete a test of manual dexterity, for example, was longer for middle-aged adults (40–60 yr) than young adults (18–36 yr) despite the two groups exhibiting similar levels of hand strength (Marmon et al. 2011). The age-associated differences in muscle function are evident at the level of single motor unit activity, as indicated by decreases in discharge rate modulation when older adults perform various tasks, such as reduced instantaneous discharge rates for motor units in tibialis anterior during ballistic contractions with dorsiflexor muscles (Klass et al. 2008), lower peak discharge rates during maximal voluntary contractions (MVCs) with the first dorsal interosseus and tibialis anterior muscles (Christie and Kamen 2010; Kamen et al. 1995; Knight and Kamen 2008), a lower incidence of double discharges for motor units in tibialis anterior during ramp isometric contractions with dorsiflexor muscles (Christie and Kamen 2010), compressed discharge rate modulation in first dorsal interosseus (Barry et al. 2007), and less modulation of discharge rate in first dorsal interosseus when tracking sinusoidal waveforms with the index finger (Knight and Kamen 2007).
It has recently been shown that the discharge characteristics of the same motor unit in the biceps brachii that was recruited during a sustained isometric contraction when pulling up against a rigid restraint were influenced by the relative target force in young adults (Riley et al. 2008a) but not in old adults (Pascoe et al. 2011). The motor units of young adults discharged action potentials either repetitively or intermittently depending on the difference between the target force and recruitment threshold force (Riley et al. 2008a), whereas the motor units of old adults always discharged action potentials repetitively at recruitment irrespective of the difference between the two forces (Pascoe et al. 2011). Additionally, the mean and variability of the discharge times for the first five interspike intervals (ISIs) at recruitment did not differ between the two target force conditions for old adults, whereas it did for young adults. Because these discharge characteristics were observed in the same motor units, there were likely greater differences in synaptic input received by the motoneuron pool across the two target force differences for the young adults.
In contrast to the preceding observations that were based on experiments in which participants exerted a force against a rigid restraint, almost nothing is known about the influence of age on motor unit activity when supporting an inertial load. It is known, however, that load compliance influences reflex responsiveness (Akazawa et al. 1983; Baudry et al. 2009a; Maluf et al. 2007; Perreault et al. 2008) and that motor unit recruitment threshold force, rate of recruitment, mean discharge rate, and discharge variability are all influenced by load compliance (Baudry et al. 2009b; Mottram et al. 2005; Tax et al. 1989, 1990; Theeuwen et al. 1994). Moreover, both reflex responses (Corden and Lippold 1996; Earles et al. 2001; Mynark and Koceja 2001; Wolfarth et al. 1997) and motor unit properties (Barry et al. 2007; Erim et al. 1999; Galganski et al. 1993; Kamen et al. 1995; Semmler et al. 2000; Vaillancourt et al. 2003) change with advancing age. The purpose of the present study was to compare the discharge characteristics of biceps brachii motor units when young and old adults performed steady, submaximal contractions while the arm supported an inertial load. To provide insight on age-associated adaptations in the integration of synaptic input by motoneurons, the approach focused on the discharge characteristics of the motor units when they were recruited during sustained contractions with different inertial loads. Because of the greater reliance by old adults on agonist-antagonist coactivation rather than modulation of spinal reflex pathways when confronted with a change in load compliance (Baudry et al. 2010), the hypothesis was that old adults would exhibit less modulation of motor unit discharge across the two inertial loads than young adults.
METHODS
Sixteen young (28.0 ± 3.8 yr, range 23–37 yr; 13 men, 3 women) and 14 old (75.1 ± 3.9 yr, range 66–81 yr; 12 men, 2 women) adults who were free from cardiovascular and neurological disorders volunteered for the study and participated in one to three experimental sessions. Informed consent was obtained from all participants, and the experimental procedures were approved by the Institutional Review Board at the University of Colorado (Protocol No. 0110.23) and were in accordance with the Declaration of Helsinki.
Experimental setup.
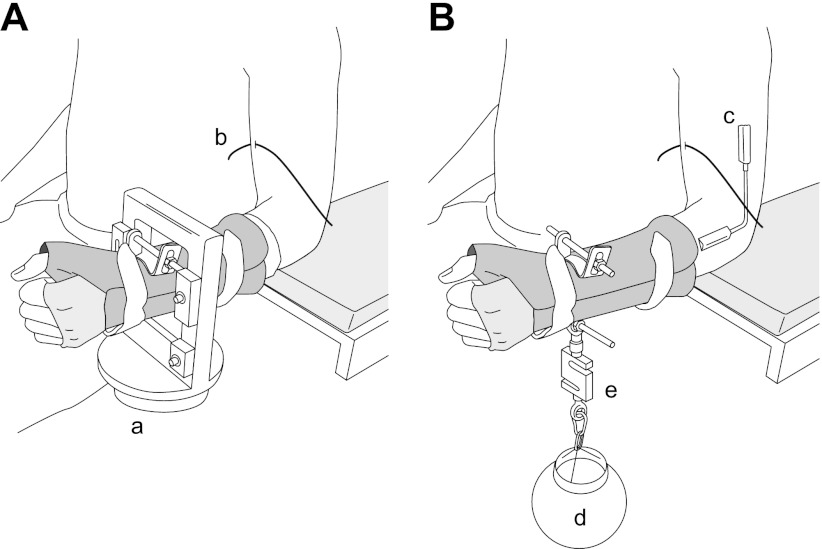
Subjects were seated upright in an adjustable chair with the left upper arm vertical and slightly abducted from the trunk (Fig. 1). The elbow was flexed to 1.57 rad and secured in a padded brace. The hand and forearm were placed in a modified wrist-hand orthosis (Orthomerica, Newport Beach, CA) and oriented in a neutral position midway between supination and pronation. The upward force exerted by the elbow flexor muscles was measured with a force transducer (900-N range, 182 N/V; model JR-3, Woodland, CA) that was attached securely to the orthosis at the level of the wrist (Fig. 1A). Visual feedback of elbow flexor force was provided on a computer monitor (43.2 cm) located at eye level ∼1.2 m in front of the subject. Force was digitized with a Power 1401 [Cambridge Electronic Design (CED), Cambridge, UK] at 200 samples/s and stored on a computer.
Fig. 1.
Position of the arm in the 2 experimental setups. A: position of the arm when pulling against a rigid restraint. Subjects were seated with the arm slightly abducted from the body and the elbow resting in a padded support. Vertical force was measured as the elbow flexor muscles contracted and the left wrist pulled up against a force transducer (a). A subcutaneous wire electrode (b) was placed over the short head of the biceps brachii muscle. B: position of the arm when supporting an inertial load. An electrogoniometer (c) was placed on the lateral aspect of the elbow to provide subjects with visual feedback of elbow angle. The task required subjects to maintain a constant elbow angle of 1.57 rad while a load (d) was hung from the wrist. A strain gauge force transducer (e) was placed in series with the load.
Participants were required to keep the elbow joint at a constant angle while supporting a mass (lead shot) placed in a plastic spherical container. The mass was placed in series with a force transducer (SBO-200, Transducer Techniques, Temecula, CA) and attached to the orthosis (Fig. 1B). The weight of the mass and transducer was set equal to a target force that depended on the recruitment threshold of an identified motor unit. Elbow joint angle was measured with an electrogoniometer (SG110 and K800, Biometrics, Cwmfelinfach, UK) attached to the lateral aspect of the elbow joint. The output from the electrogoniometer was displayed on the monitor in front of the subject and stored on a computer. The force and elbow angle signals were digitized with a Power 1401 (CED) at 200 samples/s and stored on a computer.
Electromyographic recordings.
Single motor unit recordings were obtained from the short head of biceps brachii with branched bipolar electrodes (Fig. 1; stainless steel, 50-μm diameter, Formvar insulated; California Fine Wire, Grover Beach, CA) (Enoka et al. 1988; Gydikov et al. 1986; Mottram et al. 2005). The electrode comprised two insulated wires that were glued together with three ∼1-mm regions of the insulation removed; two regions were on one wire, separated by 3 mm, and a single region was on the other wire positioned between the two regions of the same wire. The lateral margin of the short head of biceps brachii and the intermarginal septum of the two heads were identified, and a disposable hypodermic needle (25 gauge, 3.91 cm) was used to insert the wires subcutaneously across the muscle belly of the short head of biceps brachii without penetrating the muscle fascia and approximately perpendicular to the presumed orientation of the muscle fibers. The needle was removed prior to motor unit activity recording. A reference electrode was placed on the lateral epicondyle of the ipsilateral humerus. Single-motor unit recordings were amplified (×5,000) and band-pass filtered (0.3–8.5 kHz; S-series, Coulbourn Instruments, Allentown, PA). The motor unit signal was sampled at 20k samples/s with a Power 1401 (CED) and stored on a computer (Spike2, v.5.20, CED).
Surface electromyographic (EMG) recordings were obtained with a bipolar configuration of circular silver-silver chloride electrodes (8-mm diameter) placed on one side of the innervation zone for the short and long heads of biceps brachii and the lateral head of triceps brachii (interelectrode distance of ∼20 mm). Reference electrodes were placed over the acromion process of the ipsilateral scapula. Smaller electrodes (4-mm diameter) were placed over the brachioradialis muscle. The surface EMG signals were amplified (×1,000), band-pass filtered (13 Hz–1 kHz; S-series, Coulbourn Instruments), sampled at 5k samples/s, and stored on a computer (Spike2, v.5.20, CED).
Protocol.
The experimental protocol replicated previous studies with young (Riley et al. 2008a) and old (Pascoe et al. 2011) adults but also involved an inertial load hung from the wrist. The following six tasks were performed with the elbow flexor muscles of the left arm of the volunteers in each experimental session: 1) assessment of the MVC force; 2) identification of a single motor unit; 3) measurement of the recruitment and derecruitment thresholds of the motor unit; 4) performance of steady contractions while supporting a mass that was less than the recruitment threshold force of the motor unit; 5) evaluation of the recruitment and derecruitment thresholds of the motor unit immediately after the final steady contraction; and 6) a final MVC with the elbow flexor muscles. With the exception of task 4, which involved the inertial load (Fig. 1B), all tasks were performed with the elbow flexor muscles pulling against the rigid restraint (Fig. 1A). Each experiment lasted ∼2 h.
The experimental session began with a minimum of two MVCs in the direction of elbow flexion and at least one in the direction of elbow extension. The MVC task involved increasing the force from zero to maximum over 3 s and then holding the maximum for a further 2 s. Subjects rested for 90–120 s between trials. When the peak MVC forces for two elbow flexor trials were within 5% of each other, the larger of the two values was recorded as the maximum and used as a reference value for the recruitment threshold force of the motor unit. Otherwise, additional trials were performed until the 5% criterion was met. Efforts that the subject did not consider to be maximal were rejected, and the visual gain of the force feedback was varied across trials to minimize the subjects' awareness of differences in performance.
Motor units were identified in the subcutaneous EMG signal by asking subjects to increase elbow flexor force from rest to 60% MVC force at a constant rate in ∼10 s and then to relax during the subsequent 10 s to produce a triangular force profile. The location of the recording sites was adjusted by pulling on the exposed ends of the electrode to obtain the greatest signal-to-noise ratio. Once a candidate unit was identified, subjects performed three additional ramp contractions separated by 60–90 s of rest. The recruitment threshold was estimated during the experiment by noting the force at the end of the third ISI and averaging the values from four ramp contractions in which the coefficient of variation (CV) for recruitment threshold force was ≤10%. A target force that was less than the recruitment threshold force of the motor unit by either a prescribed small (∼10%) or large (∼15%) amount was determined and converted to an inertial load (kg). Prior to removal of the wrist from its attachment to the force transducer (Fig. 1A), an electrogoniometer (SG110, Biometrics) was attached to the elbow with adhesive tape and a reference angle of 1.57 rad was established.
Subsequently, one investigator returned the subject's arm to the reference angle (1.57 rad) and a second investigator attached the load to the orthosis (Fig. 1B). To avoid transient recruitment of the motor unit, the load was transferred to the subject slowly (∼5 s). The subject was instructed to maintain the target elbow angle (1.57 rad) as steadily as possible by matching the signal from the electrogoniometer to a target line on the monitor until instructed to relax. The task was terminated when the motor unit became active and discharged action potentials for ∼120 s. After 15 min of rest (Mottram et al. 2005), the contraction with the other load was performed. The presence of the same motor unit after each steady contraction was later verified off-line by comparing waveforms with Spike2 software (CED).
Data analysis.
Template matching with Spike2 software was performed off-line to discriminate individual motor unit action potentials. Waveforms were considered to belong to the same motor unit on the basis of amplitude, duration, and shape. The accuracy of the discrimination was verified by close visual inspection of each discriminated action potential and by review of the ISI; intervals >250 ms [<4 pulses/s (pps); n = 762, 3.6% of discharges] or <20 ms (>50 pps; n = 11, 0.02% of discharges) were excluded from the analysis.
Recruitment threshold force was determined with an algorithm that advanced a 500-ms window in 1-ms steps across the discharge times of the motor unit until the CV for ISI in the window was <50% (Moritz et al. 2005). The force corresponding to the time of the first discharge in the window was taken as the recruitment threshold force. The same window-sliding method was used to define the derecruitment threshold force of the motor unit as the time corresponding to the final discharge in the 500-ms window. Discharge rate and the CV for ISI were determined for the 500-ms window at recruitment and derecruitment. The rate of change in force was based on the slope of a linear trend for the force trajectory.
Each train of action potentials during the steady contraction was divided into five epochs of equal duration. The discharge rates were averaged across the entire 20% interval, and the CV for ISI (standard deviation/mean ISI × 100) was calculated from the first five ISIs in each 20% interval. The CVs (standard deviation/mean angle or force × 100) were also calculated for elbow angle and the force measured with a transducer placed in series with the load. The time to recruitment was defined as the time from complete load support to the first action potential discharged by the isolated motor unit.
Surface EMG values are reported as the root-mean-square amplitude of the signal normalized to the value recorded during a 500-ms epoch centered about the peak MVC force. Coactivation ratios were quantified as the quotient of the averaged, rectified, and normalized EMG values for the elbow extensor (lateral head of triceps brachii) relative to the pooled average of the elbow flexors (brachioradialis, short and long head of biceps brachii).
Statistical analysis.
Unpaired t-tests and repeated-measures ANOVAs were used to compare the MVC forces between young and old adults and to assess changes with time. The relations between recruitment threshold and discharge characteristics were characterized with linear regression analyses. Multiple three-factor, repeated-measures ANOVAs compared recruitment and derecruitment forces, rates of force development, discharge rates, and CV for ISI between young and old adults during ramp contractions (between-subjects factor) and before and after the steady contractions (within-subject factor). Multiple repeated-measures, two-way ANOVAs were used to compare target force difference, time to motor unit recruitment, duration of motor unit discharge, contraction duration, load, target force, and mean discharge rate and CV for ISI between the young and old adults (between-subjects factor) and the two target force differences (within-subjects factor). A χ2 analysis was used to compare discharge patterns (repetitive vs. intermittent) between young and old adults. The two patterns of motor unit discharge (repetitive and intermittent) were compared by examining discharge characteristics with unpaired t-tests.
The dependent variables were also compared with data from previous studies on young (Riley et al. 2008a) and old (Pascoe et al. 2011) adults in which the participants pulled against a rigid restraint (Fig. 1A). The data were compared with repeated-measures, three-way ANOVAs (between-subjects factors: age and load type; within-subjects factor: target force difference). Repeated-measures, three-way ANOVAs were used to compare the discharge rate and CV for ISI between the young and old adults (between-subjects factor) for the two target forces and across 20% epochs of discharge duration (within-subject factors). Post hoc analyses with paired-samples t-tests were used when appropriate to compare the mean discharge rate and CV for ISI between each 20% epoch of discharge duration. A repeated-measures, four-way ANOVA was used to compare surface EMG amplitude between the young and old adults (between-subjects factor) across the four muscles for the two target force difference tasks, from the first to the last one-third of the contraction duration (within-subject factors). A repeated-measures, three-way ANOVA was also used to compare CV for force between the young and old adults (between-subjects factor) for the two target force difference tasks, from the first to last one-third of the contraction duration (within-subject factors). An α level of P < 0.05 denoted significant differences. All statistical analyses were performed with SPSS (v.16.0, Chicago, IL). Data are presented as means ± SD in the text and Tables 1 and 2 and as means ± SE in Figs. 4 and 5.
Table 1.
Rates of change in force during ramp contractions and corresponding motor unit characteristics at recruitment and derecruitment
| Rate of Change in Force, % MVC/s |
Threshold Force, % MVC |
Discharge Rate, pps |
CV for ISI, % |
|||||
|---|---|---|---|---|---|---|---|---|
| Up | Down | Recruitment | Derecruitment | Recruitment | Derecruitment | Recruitment | Derecruitment | |
| Old (n = 20) | 5.6 ± 1.7 | 6.2 ± 2.4 | 25.9 ± 12.6 | 14.8 ± 8.2† | 11.0 ± 3.4 | 8.64 ± 2.0† | 20.3 ± 4.6 | 22.6 ± 5.8† |
| Young (n = 20) | 5.0 ± 1.4 | 5.5 ± 1.9 | 30.0 ± 10.9 | 22.9 ± 11.7† | 13.6 ± 2.8* | 9.57 ± 1.8*† | 22.0 ± 4.6 | 24.7 ± 3.9† |
Values are means ± SD. Discharge rate and coefficient of variation for interspike interval (CV for ISI) were calculated from first 5 ISIs at recruitment. MVC, maximal voluntary contraction; pps, pulses per second.
P ≤ 0.013 compared with old adults;
P ≤ 0.004 compared with recruitment.
Table 2.
Task and discharge characteristics of biceps brachii motor units at recruitment when old and young adults performed isometric contractions with elbow flexors either against a rigid restraint or supporting an inertial load at two target forces
| Old |
Young |
|||
|---|---|---|---|---|
| Rigid | Inertial | Rigid | Inertial | |
| Recruitment threshold force, % MVC | 25.4 ± 16.6 | 26.1 ± 12.4 | 32.8 ± 9.7 | 30.0 ± 10.9 |
| Target force, % MVC | ||||
| Small | 18.4 ± 7.9 | 15.5 ± 9.3 | 27.1 ± 10 | 17.5 ± 6.7 |
| Large | 13.5 ± 7.0* | 9.24 ± 7.2* | 22.3 ± 10* | 11.6 ± 5.1* |
| Target force difference, % MVC | ||||
| Small | 7.05 ± 4.2 | 10.7 ± 4.2 | 5.72 ± 2.0 | 12.7 ± 4.4 |
| Large | 11.9 ± 5.2* | 17.0 ± 6.9* | 10.5 ± 2.4* | 18.4 ± 6.2* |
| Time to onset, s | ||||
| Small | 17.9 ± 27 | 28.5 ± 59 | 22.5 ± 22 | 17.4 ± 28 |
| Large | 160 ± 145* | 202 ± 265* | 37.1 ± 50 | 172 ± 187* |
| Contraction duration, s | ||||
| Small | 84.4 ± 29 | 185 ± 219 | 138 ± 21 | |
| Large | 223 ± 147* | 325 ± 266* | 295 ± 195* | |
| Mean discharge rate, pps | ||||
| Small | 8.3 ± 2.5 | 9.1 ± 2.9 | 13.2 ± 3.5 | 10.9 ± 3.3 |
| Large | 8.4 ± 3.3 | 9.3 ± 4.8 | 13.9 ± 4.3 | 8.6 ± 2.5* |
| CV for ISI, % | ||||
| Small | 26.3 ± 15 | 21.0 ± 9.8 | 18.7 ± 7.9 | 27.3 ± 13 |
| Large | 24.0 ± 13 | 23.7 ± 9.1 | 35.0 ± 10* | 27.9 ± 12 |
Values are means ± SD. Data for the rigid restraint condition were obtained from Pascoe et al. (2011) for the old adults and from Riley et al. (2008a) for the young adults. “Small” and “large” refer to the difference between the target force and recruitment threshold force of the motor unit.
P < 0.007 compared with small target force difference for the same age and type of load.
Fig. 4.
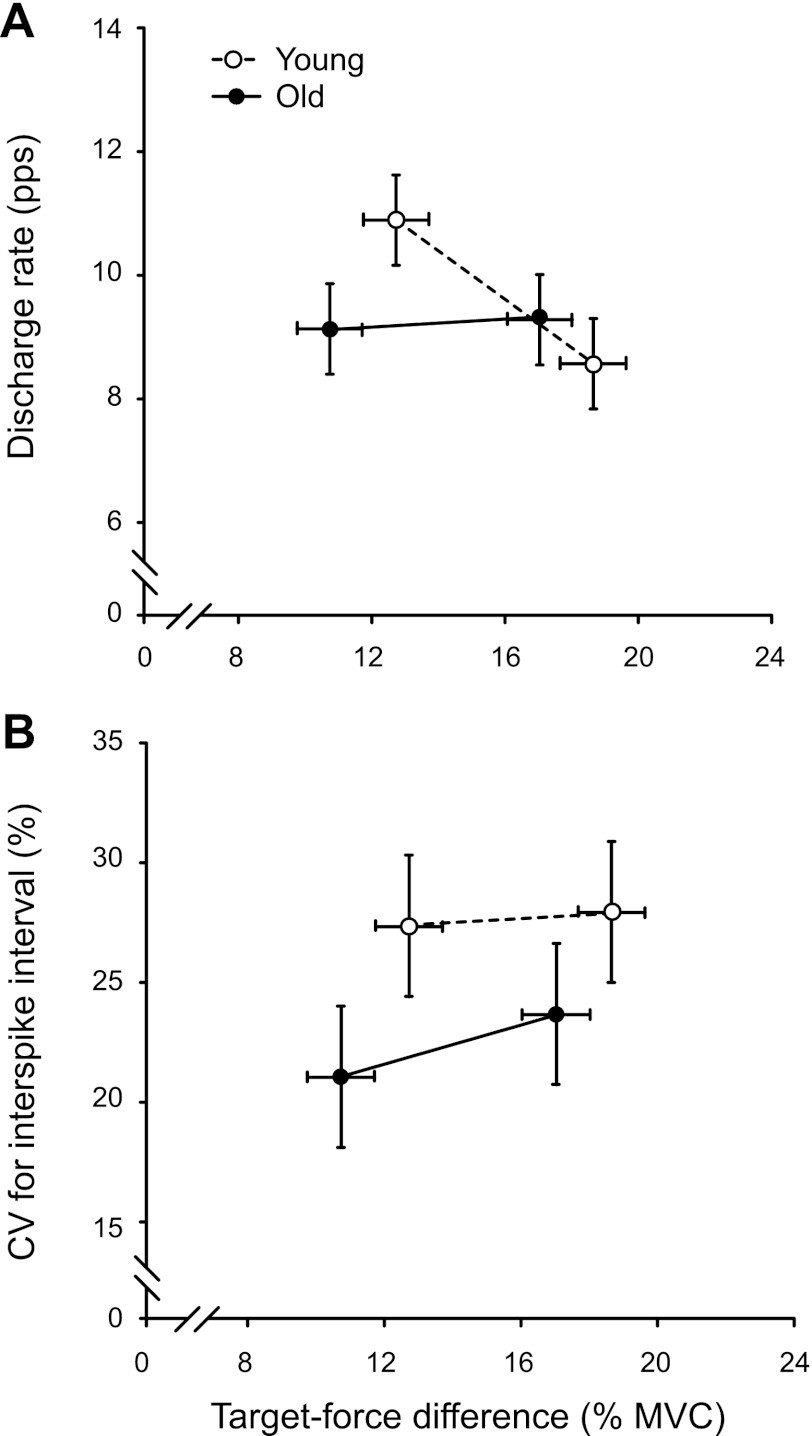
Motor unit discharge characteristics at recruitment when the elbow flexor muscles supported a mass: discharge characteristics at recruitment for 20 motor units recorded from the biceps brachii muscle from old adults and 20 motor units from young adults. A: mean discharge rate for the first 5 interspike intervals (ISIs) was less for the large difference between target force and recruitment threshold force in young adults only (age × target force difference interaction: P = 0.036). B: coefficient of variation (CV) for ISI did not differ between young and old adults (P = 0.07) or between the small and large differences between target force and recruitment threshold force (P = 0.43).
Fig. 5.
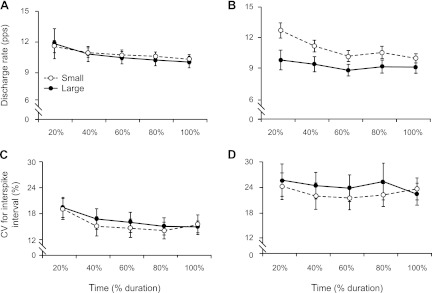
Motor unit discharge characteristics during the steady contraction when supporting a mass with the elbow flexor muscles. A and B: mean discharge rate for 20 motor units from old adults (A) and 20 motor units from young adults (B) for the small and large differences between target force and recruitment threshold force [%maximal voluntary contractions (MVC)] for each 20% epoch of discharge duration. C and D: CV for the first 5 ISIs recorded at the beginning of each 20% epoch during the sustained contraction for old (C) and young (D) adults.
RESULTS
The results comprise recordings from 40 motor units in the short head of biceps brachii, 20 units from old and 20 units from young adults, when they used the elbow flexor muscles to support a mass that was less than the recruitment threshold force of an isolated motor unit (Fig. 1B). A single motor unit was recorded in 22 subjects (12 young, 10 old), 2 motor units were recorded in 6 subjects (4 young, 2 old), and 3 motor units were recorded in 2 subjects (both old). The discharge of each motor unit was recorded during two contractions that differed in the size of the mass hung from the wrist relative to the recruitment threshold force of the isolated motor unit. Subjects performed two to four trials of the MVC task to meet the between-trial criterion of a 5% difference. Initial MVC forces for the elbow flexor and extensor muscles were less for the old adults (200 ± 67 N and 116 ± 53 N, respectively) than the young adults (280 ± 91 N and 170 ± 76 N; P < 0.012). The decline in elbow flexor MVC force (old: 7 ± 23%, young: 9 ± 21%) by the end of the protocol (old: 185 ± 70 N, young: 251 ± 89 N; main effect for time: P = 0.003) was similar for the two groups (age × time interaction: P = 0.295).
Recruitment and derecruitment thresholds during ramp contractions.
Motor unit recruitment thresholds extended across a wide range of forces in both old (7.5–47.0% MVC) and young (14.0–52.5% MVC) adults. The average CV for the recruitment threshold forces during the four ramp contractions was similar for old and young adults (6.1 ± 3.3%, P = 0.45). The absolute rates of change in force did not differ between the up and down phases of the ramp contractions for either old (P = 0.06) or young (P = 0.084) adults. Average recruitment threshold force did not differ between old and young adults (main effect for age: P = 0.06; Table 1) but was significantly reduced (10 ± 10%) when measured after the steady contractions (main effect for time: P = 0.001). Derecruitment thresholds, which were identified for 37 of the 40 motor units (19 old, 18 young; Table 1), were less than recruitment threshold forces (P < 0.001) for both old and young adults (threshold force × age interaction: P = 0.120; Table 1). Discharge rates at recruitment were significantly greater than those at derecruitment (P < 0.001) and were 20% less for old adults (main effect for age: P = 0.001; Table 1). The CV for ISI at recruitment was significantly less than that at derecruitment (P = 0.017) but did not differ between old and young adults (main effect for age: P = 0.095; Table 1). Correlation analysis revealed a positive linear trend in mean discharge rate at recruitment (r = 0.506, P = 0.023) during the ramp tasks as a function of recruitment threshold force. The only statistically significant difference between the two groups of participants during the ramp contractions, therefore, was the lower mean discharge rates for the old adults at recruitment and derecruitment.
Recruitment characteristics during steady contractions while supporting a mass.
The two target forces (16.5 ± 8.0 and 10.4 ± 6.3% MVC; P < 0.001) did not differ between old and young adults (main effect for age: P = 0.340). However, the absolute loads supported were less for old adults with both the small (3.1 ± 2.0 kg) and large (1.8 ± 1.5 kg) target force differences than for young adults (4.9 ± 2.1 kg and 3.2 ± 1.5 kg, respectively; main effect for age: P = 0.009). The difference (P < 0.001) between the recruitment threshold force and target force for the small (11.7 ± 4.4% MVC) and large (17.8 ± 6.5% MVC) differences was similar for old and young adults (main effect for age: P = 0.297). The time to recruitment was longer for the large target force difference (187 ± 227 s) than for the small target force difference (23 ± 46 s; P < 0.001) but did not differ between old and young adults (main effect for age: P = 0.586).
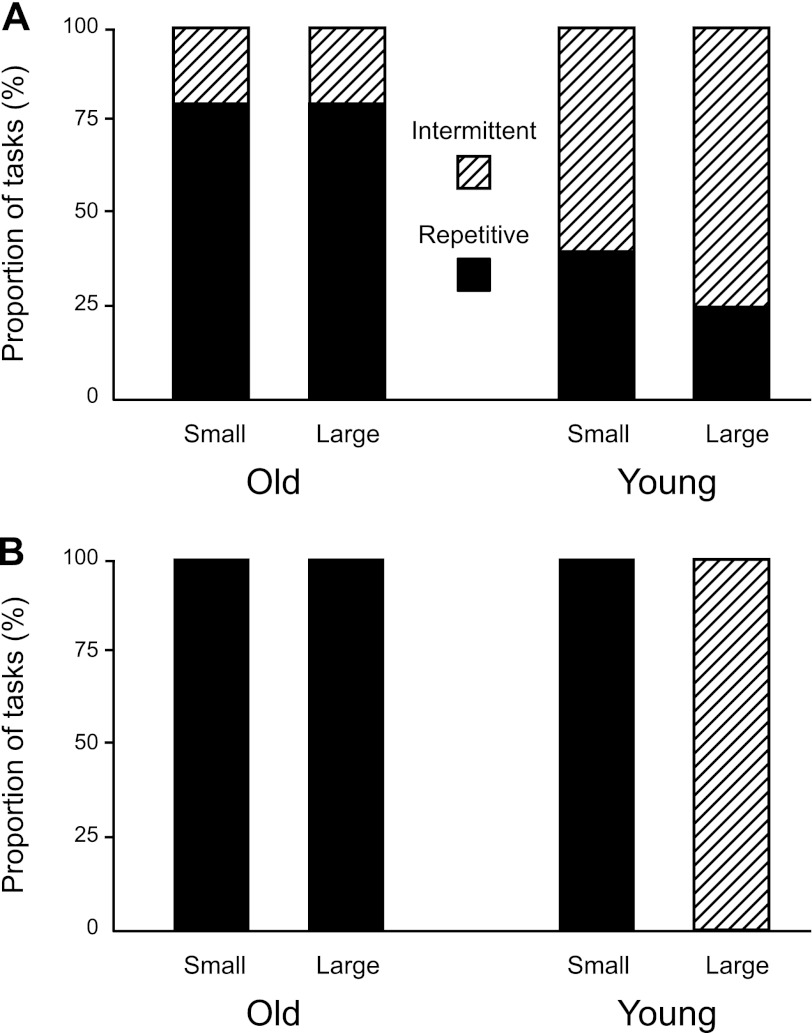
Motor units discharged action potentials at recruitment with one of two patterns: intermittent (Fig. 2, A and B) or repetitive (Fig. 2, C and D). The discharge pattern was characterized as intermittent when the motor unit stopped discharging action potentials for >1 s after it had been recruited. The discharge pattern exhibited by a motor unit was often similar for both target force differences. For example, Fig. 2 shows one motor unit from a young adult (recruitment threshold force = 46.8% MVC) that discharged action potentials intermittently for both small (15.7% MVC force; Fig. 2A) and large (28.3% MVC force; Fig. 2B) target force differences and another motor unit from an old adult (recruitment threshold force = 20.1% MVC) that discharged action potentials repetitively at recruitment for both the small (11.4% MVC force; Fig. 2C) and large (15.4% MVC force; Fig. 2D) target force differences. The old adults had a greater proportion (χ2 = 18.34, df = 1, P < 0.01; Fig. 3A) of motor units that discharged action potentials repetitively at recruitment compared with young adults.
Fig. 2.
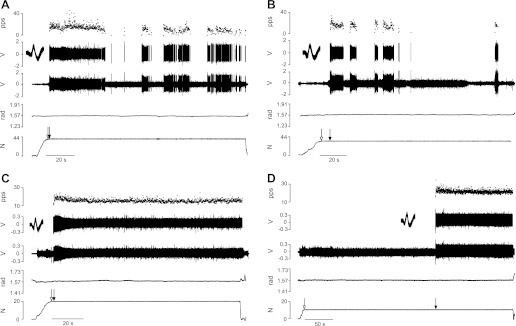
Motor units discharged action potentials either intermittently or repetitively at recruitment. A–D, from top to bottom: instantaneous discharge rate of the motor unit (pulses per second, pps), discriminated action potentials with waveform overlay, interference EMG from the wire electrode, electrogoniometer measurement of elbow angle, and signal from the force transducer in series with the load. The 4 panels show the 2 discharge patterns (intermittent and repetitive) observed when there were small and large differences between the target force and the recruitment threshold force of the motor unit. The motor unit from a young adult shown in A and B discharged intermittently for both small (A) and large (B) differences between the 2 forces. The motor unit from an old adult shown in C and D discharged repetitively for both small (C) and large (D) target-force differences. Open arrows indicate the start of the task; filled arrows denote the onset of motor unit activity.
Fig. 3.
Discharge pattern of biceps brachii motor units at recruitment when old and young adults sustained an isometric contraction with the elbow flexor muscles: comparison of motor unit discharge pattern while supporting an inertial mass as observed in the present study (A) and while pulling up against a rigid restraint as reported previously (B). A: a greater proportion of motor units for old adults discharged action potentials repetitively at recruitment for both small and large target force differences when the task was to support a mass (high-compliance load) hung from the wrist. B: motor units of old adults always discharged action potentials repetitively at recruitment for both small and large target force differences when exerting a force against a rigid restraint (Pascoe et al. 2011), whereas the pattern exhibited by young adults depended on the target force difference (Riley et al. 2008a).
Mean discharge rate at recruitment in the present study was greater (P < 0.0001) for old adults when the motor unit discharge was intermittent (13.4 ± 6.5 pps) than when it was repetitive (8.15 ± 2.0 pps), whereas for young adults mean discharge rate was greater (P = 0.024) when the discharge was repetitive (11.3 ± 3.5 pps) than when it was intermittent (8.97 ± 2.6 pps). Furthermore, CV for ISI was less (P = 0.035) for the repetitive pattern (old: 20.6%, young: 21.8%) than for the intermittent pattern (old: 29.2%, P = 0.02; young: 30.4%). Consequently, the initial discharge characteristics suggested that the motor units of young adults were closer to recruitment threshold when the discharge was intermittent, but the behavior was less consistent for the old adults.
A significant interaction between age and target force difference (P = 0.036; Table 2) indicated that mean discharge rate at recruitment differed for the young adults between the small (10.9 ± 3.3 pps) and large (8.6 ± 2.5 pps) target force differences when supporting an inertial load, whereas there was no difference for old adults (9.1 ± 2.9 and 9.3 ± 4.8 pps, respectively) (Table 2, Fig. 4A). The CV for ISI when supporting an inertial load did not differ between the small (24.2 ± 11.8%) and large (25.8 ± 10.5%; main effect for target force difference: P = 0.426) target force differences or between the old (22.4 ± 9.4%) and young (27.6 ± 12.3%; main effect for age: P = 0.071; Fig. 4B) adults. Regression analyses revealed that mean discharge rate at recruitment for old adults (r = 0.503, P = 0.024) and CV for ISI at recruitment for young adults (r = 0.595, P = 0.006) increased as a function of recruitment threshold force, but only for the small target force difference. The only difference between the two age groups in discharge characteristics at recruitment when supporting an inertial load, therefore, was that the young adults varied mean discharge rate across the two target forces.
Adjustments during steady contractions.
Surface EMG amplitude was greater for all muscles during the steady contraction at the small target force difference (main effect for target force difference: P < 0.001) and increased from the first (16.3 ± 10.0% maximal EMG) to the last (20.1 ± 13.7% maximal EMG) one-third of the contraction duration for the elbow flexor muscles (main effect for time: P < 0.001) but not the elbow extensor muscle (triceps brachii: P = 0.087). Additionally, a nonsignificant age × muscle group interaction (P = 0.208) indicated that the surface EMG amplitude of both the elbow flexor and extensor muscles was greater for old adults (18.3 ± 10.7% and 13.7 ± 12.5%, respectively) relative to young adults (12.3 ± 5.4% and 4.4 ± 3.0%, respectively). However, the coactivation ratio did not reach statistical significance (main effect for age: P = 0.063) between old (52.7 ± 27.9%) and young (35.1 ± 22.9%) adults. Nonetheless, the coactivation ratio decreased from the first (43.6 ± 27.0%) to the last (40.6 ± 25.2%) one-third of the contraction duration (main effect for time: P = 0.004) and was less for small (38.9 ± 24.3%) than large (45.4 ± 27.8%, main effect for target force difference: P < 0.001) target force difference.
The absence of a statistically significant interaction between age, target force difference, and time (P = 0.946) indicated that the CV for the force applied to the load for the small (1.4 ± 1.0%) and large (2.5 ± 2.4%) target force differences differed (main effect for target force difference: P < 0.001) to the same extent for the two groups of subjects from the first to the last one-third of the contraction duration. This finding indicates that there was no difference between the two age groups in force steadiness during the contractions.
Motor unit discharge times were recorded for 116 ± 21 s after recruitment during the steady contractions. Mean discharge rate across the five epochs (mean of the means) did not differ between old (10.8 ± 2.1 pps) and young (10.2 ± 2.5 pps) adults during the steady contraction (main effect for age: P = 0.650) and decreased (main effect for time: P < 0.001) similarly for the two groups (time × age interaction: P = 0.405). A significant age × force difference interaction (P = 0.026) indicated no difference for old adults in discharge rate between the small (10.8 ± 1.5 pps) and large (10.7 ± 2.5 pps) target force difference (Fig. 5A), whereas there was a difference for young adults (small: 11.0 ± 2.2 pps, large: 9.3 ± 2.6 pps; Fig. 5B). The interaction between age and target force difference and time was not significant (P = 0.335).
The average CV for ISI (mean of the means) was less for old (16.0 ± 6.7%) than for young (23.5 ± 11.0%) adults during the steady contractions (main effect for age: P = 0.020) but decreased (main effect for time: P < 0.001) similarly for both groups (time × age interaction: P = 0.217). A significant interaction between age and target force difference (P = 0.039) indicated that there was no difference in discharge variability for old adults between the small (15.7 ± 6.9%) and large (16.4 ± 6.6%) target force differences (Fig. 5C), whereas there was a difference for young adults (small: 22.8 ± 10.2%, large: 24.4 ± 12.1%; Fig. 5D). These findings indicate that the two groups adjusted mean discharge rate and discharge variability similarly during the steady contractions, but only the young adults modulated the discharge characteristics across the two target forces.
Comparison with a rigid restraint.
The results from the present study were compared with data from previous studies using the identical protocol in old (Pascoe et al. 2011; n = 27) and young (Riley et al. 2008a; n = 12) adults but with a task that required the subjects to exert a force against a rigid restraint (Fig. 1A; Table 2). The target force difference was similar for the two age groups and load types (age × load type interaction: P = 0.164). The duration of the steady contractions for both load types was briefer when the task involved a small (120 ± 119 s) than a large (242 ± 207 s) target force difference (main effect: P < 0.001).
The time to recruitment across load types was briefer for the small (21.2 ± 37.0 s) relative to the large (155 ± 189 s) target force difference (main effect: P < 0.001) and similar for old and young adults (age × load type interaction: P = 0.396). Additionally, motor units were recruited sooner for tasks involving a rigid restraint (70.9 ± 80.6 s vs. 105 ± 136 s; main effect for load type: P = 0.049). A significant age × load type interaction (P = 0.001) indicated that old adults did not exhibit a difference in mean discharge rate for the first five ISIs at recruitment for the two target forces with either a rigid restraint (8.4 ± 2.9 pps) or an inertial load (8.8 ± 3.9 pps), whereas there was a difference for young adults (rigid restraint: 13.5 ± 3.9 pps, inertial load: 9.7 ± 2.9 pps). A significant interaction between age and target force difference (P = 0.01) for the CV for ISI indicated no difference for old adults between small (24.1 ± 13.0%) and large (23.9 ± 11.4%) target force differences, whereas the CV for ISI differed for young adults (small: 24.1 ± 12.0%, large: 30.6 ± 11.4%). Additionally, a significant age × target force difference × load type interaction (P = 0.002) indicated that CV for ISI did not change with target force difference for either load type for old adults, whereas for young adults it was greater for the large target force difference for tasks involving a rigid restraint but there was no target force difference for the inertial load (Table 2). These data indicate that the modulation of discharge characteristics at recruitment by young adults, but not old adults, differed across the two load types.
DISCUSSION
The main findings of the present study were that motor units in biceps brachii discharged action potentials either intermittently or repetitively at recruitment when the arm supported an inertial load, the discharge pattern did not depend on the time that elapsed from the beginning of the contraction until the motor unit was recruited, the old adults exhibited the repetitive pattern more often than the young adults, and discharge rate differed across two contraction intensities for young adults but not old adults. When compared with previously published results in which the arm pulled against a rigid restraint, the most striking difference was the less distinct distribution of discharge patterns (repetitive vs. intermittent) across the two target forces for the young and old adults in the present study.
Influence of target force difference on discharge characteristics when supporting a mass.
When each motor unit was recruited during the sustained contractions it discharged action potentials either intermittently or repetitively for both young and old adults. Although the discharge pattern was not related to the target force difference, there was a greater prevalence of the repetitive pattern for old adults compared with young adults (Fig. 3A). Moreover, discharge rate at recruitment (Table 2) and during the steady contraction (Fig. 5) was similar at the two target forces for old adults but was greater for the stronger contraction for young adults. In contrast, the variability in discharge times at recruitment was similar for the two groups of participants but was less for the old adults during the steady contraction. The discharge pattern, which influenced the variability in discharge times, was not associated with the steadiness of the contraction, as the CV for the force exerted by the elbow flexors did not differ between old and young adults and there were no differences in the CV for force between the two load types.
The lower prevalence of the intermittent pattern exhibited by the old adults is consistent with a reduced capacity to modulate discharge rate. The adaptation presumably involves both intrinsic motoneuron properties (Christie and Kamen 2010; Piotrkiewicz et al. 2007) and the magnitude and distribution of synaptic inputs (Earles et al. 2001; Eisen et al. 1996; Erim et al. 1999; Kido et al. 2004; Oliviero et al. 2006; Patten and Kamen 2000), which together influence the transformation of synaptic inputs into trains of motor unit action potentials. The present findings suggest that the integration of synaptic inputs as the membrane potential approached voltage threshold for generating action potentials differed for the two groups of subjects. When motor units exhibited the intermittent discharge pattern, for example, the initial discharge characteristics for young adults suggested that the motor units were close to recruitment threshold (low mean discharge rate and high CV for ISI), whereas those for old adults exhibited a mixed profile (high mean discharge rate and high CV for ISI). These differences presumably contributed to the reduced discharge rate modulation for old adults as reported previously for a hand muscle across multiple isometric target forces (Barry et al. 2007) and during a sinusoidal matching task (Knight and Kamen 2007).
Moreover, the reduced modulation of discharge rate for old adults was underscored by the difference in the CV for ISI between the two target force differences for young adults, but not the old adults, during the steady contractions (Fig. 5, C and D). Because the CV for ISI is greatest at forces close to recruitment threshold and declines with an increase in target force for both old and young adults (Barry et al. 2007), the absence of a target force difference in CV for ISI during the steady contraction for old adults again suggests age-associated differences in the integration of synaptic inputs at the time of motor unit recruitment when supporting an inertial load.
Influence of load type on motor unit discharge characteristics at recruitment.
The most substantial difference between the present findings on the modulation of discharge rate when supporting an inertial load and previous results in which the arm pulled against a rigid restraint was the relative distribution of the discharge patterns across the two target forces (Fig. 3B). When exerting a force against a rigid restraint, biceps brachii motor units of old adults discharged action potentials repetitively for both small and large target force differences (Pascoe et al. 2011), whereas the discharge pattern for motor units of young adults depended on the target force difference (Riley et al. 2008a). These results are consistent with indirect evidence supporting a tendency toward repetitive motor unit discharge in older adults as suggested by the decreased rate of bursts of activity in the surface EMG recordings (Riley et al. 2008b) when the elbow flexor muscles supported an inertial load and pulled up against a rigid restraint during fatiguing contractions that were sustained at 20% MVC force (Hunter et al. 2005). In contrast to the present findings, young adults varied the CV for ISI but not mean discharge rate across the two target forces with the rigid restraint, but again the old adults modulated neither discharge property across the two target forces (Table 2).
Because the different discharge patterns within each load type were observed in the same motor unit, differences in synaptic input presumably contribute to the age and load type differences. There were no differences for the biceps brachii motor units of old adults in either mean discharge rate or CV for ISI with either load type or target force (Table 2). In contrast, young adults exhibited both load type and target force differences in motor unit discharge characteristics. When supporting an inertial load, mean discharge rate at recruitment was less for the large target force difference (lower target force) relative to the small target force difference (Table 2). Based on the current-frequency relation for motoneurons (Heckman and Enoka 2012), this observation suggests that the motor units were recruited with less synaptic current when supporting a lighter mass with a weaker muscle contraction. Similarly, the CV for ISI when the young adults exerted a force against a rigid restraint was greater for the large relative to the small target force difference (Table 2), again consistent with the motor units requiring less synaptic currents to begin discharging action potentials during the weaker contraction (Person and Kudina 1972). The underlying mechanisms are likely more involved, however, because recruitment with less synaptic current should be manifest as both a decrease in mean discharge rate and an increase in discharge variability for each load type.
Because aging is associated with declines in sensory sensitivity, slowing of conduction velocity, and reductions in the amplitude of the responses evoked in spinal pathways (Burke and Kamen 1996; Chung et al. 2005; Corden and Lippold 1996; Kido et al. 2004; Nadler et al. 2002; Tang and Woollacott 1999), old adults modulate afferent feedback less than young adults when performing voluntary actions and tend to rely more on adjusting levels of agonist and antagonist coactivation (Baudry et al. 2010). For example, old adults limit the gain of responses arising from the muscle spindle relative to young adults in response to changes in posture and accompanying muscle contractions (Angulo-Kinzler et al. 1998; Tsuruike et al. 2003) by modulating presynaptic inhibition of Ia afferents less when proceeding from rest to a submaximal contraction (Butchart et al. 1993; Earles et al. 2001; Morita et al. 1995) and when changing from a supine to a standing posture (Koceja and Mynark 2000), and they use less reciprocal Ia inhibition during movement (Hortobágyi et al. 2006; Kido et al. 2004). Moreover, afferent feedback also differs with load type (Akazawa et al. 1983; Perreault et al. 2008), as indicated by heightened sensitivity of stretch reflex pathways (Maluf and Enoka 2005) and an increase in presynaptic Ia inhibition (Baudry and Enoka 2009) during tasks that involve inertial loads. When supporting the inertial mass in the present study, therefore, the less consistent discharge pattern at recruitment was likely due to a more diverse set of synaptic inputs compared with exerting a force against a rigid restraint. This effect appears to have been less profound for the old adults because the change in discharge pattern was less frequent and is consistent with the interpretation that the motoneurons of old adults have a reduced capacity to modulate discharge rate.
In summary, the present findings indicate that motor units in biceps brachii discharged action potentials either intermittently or repetitively when recruited while supporting different inertial loads, but old adults exhibited the repetitive pattern more often than young adults. Whereas young adults modulated discharge rate across the two inertial loads, both at recruitment and during the steady contraction, old adults did not. These results indicate that the reduced ability of old adults to modulate discharge rate during submaximal voluntary contractions was evident from the moment the synaptic inputs exceeded voltage threshold for the discharge of action potentials. Previously reported results in which the arm pulled against a rigid restraint indicated that old adults did not modulate either discharge rate or discharge pattern across two target forces, whereas young adults exhibited an intermittent pattern for a lower target force and the repetitive pattern for the greater target force.
GRANTS
Awards from National Institute on Aging Grants AG-09000 to R. M. Enoka and T32 AG-000279-08 awarded to Robert Schwartz, which supported M. A. Pascoe, funded this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A.P. and R.M.E. conception and design of research; M.A.P. and J.R.G. performed experiments; M.A.P. and J.R.G. analyzed data; M.A.P., J.R.G., and R.M.E. interpreted results of experiments; M.A.P. prepared figures; M.A.P., J.R.G., and R.M.E. drafted manuscript; M.A.P., J.R.G., and R.M.E. edited and revised manuscript; M.A.P., J.R.G., and R.M.E. approved final version of manuscript.
REFERENCES
- Adam A, De Luca CJ. Recruitment order of motor units in human vastus lateralis muscle is maintained during fatiguing contractions. J Neurophysiol 90: 2919–2927, 2003 [DOI] [PubMed] [Google Scholar]
- Akazawa K, Milner TE, Stein RB. Modulation of reflex EMG and stiffness in response to stretch of human finger muscle. J Neurophysiol 49: 16–27, 1983 [DOI] [PubMed] [Google Scholar]
- Angulo-Kinzler R, Mynark R, Koceja D. Soleus H-reflex gain in elderly and young adults: modulation due to body position. J Gerontol A Biol Sci Med Sci 53: M120–M125, 1998 [DOI] [PubMed] [Google Scholar]
- Barry BK, Pascoe MA, Jesunathadas M, Enoka RM. Rate coding is compressed but variability is unaltered for motor units in a hand muscle of old adults. J Neurophysiol 97: 3206–3218, 2007 [DOI] [PubMed] [Google Scholar]
- Baudry S, Enoka R. Influence of load type on presynaptic modulation of Ia afferent input onto two synergist muscles. Exp Brain Res 199: 83–88, 2009 [DOI] [PubMed] [Google Scholar]
- Baudry S, Jordan K, Enoka RM. Heteronymous reflex responses in a hand muscle when maintaining constant finger force or position at different contraction intensities. Clin Neurophysiol 120: 210–217, 2009a [DOI] [PubMed] [Google Scholar]
- Baudry S, Maerz AH, Enoka RM. Presynaptic modulation of Ia afferents in young and old adults when performing force and position control. J Neurophysiol 103: 623–631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry S, Rudroff T, Pierpoint LA, Enoka RM. Load type influences motor unit recruitment in biceps brachii during a sustained contraction. J Neurophysiol 102: 1725–1735, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J, Kamen G. Changes in spinal reflexes preceding a voluntary movement in young and old adults. J Gerontol A Biol Sci Med Sci 51: M17–M22, 1996 [DOI] [PubMed] [Google Scholar]
- Butchart P, Farquhar R, Part NJ, Roberts RC. The effect of age and voluntary contraction on presynaptic inhibition of soleus muscle Ia afferent terminals in man. Exp Physiol 78: 235–242, 1993 [DOI] [PubMed] [Google Scholar]
- Carpentier A, Duchateau J, Hainaut K. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol 534: 903–912, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie A, Kamen G. Short-term training adaptations in maximal motor unit firing rates and afterhyperpolarization duration. Muscle Nerve 41: 651–660, 2010 [DOI] [PubMed] [Google Scholar]
- Christova P, Kossev A. Motor unit activity during long-lasting intermittent muscle contractions in humans. Eur J Appl Physiol Occup Physiol 77: 379–387, 1998 [DOI] [PubMed] [Google Scholar]
- Chung S, van Rey E, Bai Z, Rogers M, Roth E, Zhang L. Aging-related neuromuscular changes characterized by tendon reflex system properties. Arch Phys Med Rehabil 86: 318–327, 2005 [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci 63: 829–834, 2008 [DOI] [PubMed] [Google Scholar]
- Corden DM, Lippold OC. Age-related impaired reflex sensitivity in a human hand muscle. J Neurophysiol 76: 2701–2706, 1996 [DOI] [PubMed] [Google Scholar]
- de Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 329: 113–128, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O. Neural control of aging skeletal muscle. Aging Cell 2: 21–29, 2003 [DOI] [PubMed] [Google Scholar]
- Denier van der Gon JJ, ter Haar Romeny BM, van Zuylen EJ. Behaviour of motor units of human arm muscles: differences between slow isometric contraction and relaxation. J Physiol 359: 107–118, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt JE, Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol 264: 673–693, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol 95: 1717–1727, 2003 [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Brown WF. Effects of ageing on the motor unit: a brief review. Can J Appl Physiol 18: 331–358, 1993 [DOI] [PubMed] [Google Scholar]
- Earles D, Vardaxis V, Koceja DM. Regulation of motor output between young and elderly subjects. Clin Neurophysiol 112: 1273–1279, 2001 [DOI] [PubMed] [Google Scholar]
- Eisen A, Entezari-Taher M, Stewart H. Cortical projections to spinal motoneurons: changes with aging and amyotrophic lateral sclerosis. Neurology 46: 1396–1404, 1996 [DOI] [PubMed] [Google Scholar]
- Enoka RM, Robinson GA, Kossev A. A stable, selective electrode for recording single motor-unit potentials in humans. Exp Neurol 99: 761–764, 1988 [DOI] [PubMed] [Google Scholar]
- Enoka RM, Robinson GA, Kossev A. Task and fatigue effects on low-threshold motor units in human hand muscle. J Neurophysiol 62: 1344–1359, 1989 [DOI] [PubMed] [Google Scholar]
- Erim Z, Beg MF, Burke D, De Luca CJ. Effects of aging on motor-unit control properties. J Neurophysiol 82: 2081–2091, 1999 [DOI] [PubMed] [Google Scholar]
- Freund HJ, Büdingen HJ, Dietz V. Activity of single motor units from human forearm muscles during voluntary isometric contractions. J Neurophysiol 38: 933–946, 1975 [DOI] [PubMed] [Google Scholar]
- Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol 69: 2108–2115, 1993 [DOI] [PubMed] [Google Scholar]
- Garland SJ, Enoka RM, Serrano LP, Robinson GA. Behavior of motor units in human biceps brachii during a submaximal fatiguing contraction. J Appl Physiol 76: 2411–2419, 1994 [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002 [DOI] [PubMed] [Google Scholar]
- Gydikov AA, Kosarov D, Kossev A, Kostov K, Trayanova N, Radicheva N. Motor unit potentials at high muscle activity recorded by selective electrodes. Biomed Biochim Acta 45: S63–S68, 1986 [PubMed] [Google Scholar]
- Heckman CJ, Enoka RM. The motor unit. Compr Physiol 2: 2629–2682, 2012 [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, del Olmo MF, Rothwell JC. Age reduces cortical reciprocal inhibition in humans. Exp Brain Res 171: 322–329, 2006 [DOI] [PubMed] [Google Scholar]
- Hunter SK, Rochette L, Critchlow A, Enoka RM. Time to task failure differs with load type when old adults perform a submaximal fatiguing contraction. Muscle Nerve 31: 730–740, 2005 [DOI] [PubMed] [Google Scholar]
- Jesunathadas M, Marmon AR, Gibb JM, Enoka RM. Recruitment and derecruitment characteristics of motor units in a hand muscle of young and old adults. J Appl Physiol 108: 1659–1667, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen G, Sison SV, Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol 79: 1908–1913, 1995 [DOI] [PubMed] [Google Scholar]
- Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol 82: 238–248, 2004 [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol 104: 739–746, 2008 [DOI] [PubMed] [Google Scholar]
- Knight CA, Kamen G. Modulation of motor unit firing rates during a complex sinusoidal force task in young and older adults. J Appl Physiol 102: 122–129, 2007 [DOI] [PubMed] [Google Scholar]
- Knight CA, Kamen G. Relationships between voluntary activation and motor unit firing rate during maximal voluntary contractions in young and older adults. Eur J Appl Physiol 103: 625–630, 2008 [DOI] [PubMed] [Google Scholar]
- Koceja D, Mynark R. Comparison of heteronymous monosynaptic Ia facilitation in young and elderly subjects in supine and standing positions. Int J Neurosci 103: 1–17, 2000 [DOI] [PubMed] [Google Scholar]
- Maluf KS, Barry BK, Riley ZA, Enoka RM. Reflex responsiveness of a human hand muscle when controlling isometric force and joint position. Clin Neurophysiol 118: 2063–2071, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maluf KS, Enoka RM. Task failure during fatiguing contractions performed by humans. J Appl Physiol 99: 389–396, 2005 [DOI] [PubMed] [Google Scholar]
- Marmon AR, Pascoe MA, Schwartz RS, Enoka RM. Associations among strength, steadiness, and hand function across the adult life span. Med Sci Sports Exerc 43: 560–567, 2011 [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol 230: 359–370, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, Shindo M, Yanagawa S, Yoshida T, Momoi H, Yanagisawa N. Progressive decrease in heteronymous monosynaptic Ia facilitation with human ageing. Exp Brain Res 104: 167–170, 1995 [DOI] [PubMed] [Google Scholar]
- Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93: 2449–2459, 2005 [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Jakobi JM, Semmler JG, Enoka RM. Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol 93: 1381–1392, 2005 [DOI] [PubMed] [Google Scholar]
- Mynark RG, Koceja DM. Effects of age on the spinal stretch reflex. J Appl Biomech 17: 188–203, 2001 [Google Scholar]
- Nadler MA, Harrison LM, Stephens JA. Changes in cutaneomuscular reflex responses in relation to normal ageing in man. Exp Brain Res 146: 48–53, 2002 [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. Effects of aging on motor cortex excitability. Neurosci Res 55: 74–77, 2006 [DOI] [PubMed] [Google Scholar]
- Oya T, Riek S, Cresswell AG. Recruitment and rate coding organisation for soleus motor units across entire range of voluntary isometric plantar flexions. J Physiol 587: 4737–4748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe MA, Holmes MR, Enoka RM. Discharge characteristics of biceps brachii motor units at recruitment when older adults sustained an isometric contraction. J Neurophysiol 105: 571–581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten C, Kamen G. Adaptations in motor unit discharge activity with force control training in young and older human adults. Eur J Appl Physiol 83: 128–143, 2000 [DOI] [PubMed] [Google Scholar]
- Perreault EJ, Chen K, Trumbower RD, Lewis G. Interactions with compliant loads alter stretch reflex gains but not intermuscular coordination. J Neurophysiol 99: 2101–2113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person RS, Kudina L. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol 32: 471–483, 1972 [DOI] [PubMed] [Google Scholar]
- Piotrkiewicz M, Kudina L, Mierzejewska J, Jakubiec M, Hausmanowa-Petrusewicz I. Age-related change in duration of afterhyperpolarization of human motoneurones. J Physiol 585: 483–490, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley ZA, Maerz A, Litsey J, Enoka RM. Motor unit recruitment in human biceps brachii during sustained voluntary contractions. J Physiol 586: 2183–2193, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley ZA, Terry ME, Mendez-Villanueva A, Litsey JC, Enoka RM. Motor unit recruitment and bursts of activity in the surface electromyogram during a sustained contraction. Muscle Nerve 37: 745–753, 2008b [DOI] [PubMed] [Google Scholar]
- Romaiguère P, Vedel JP, Pagni S. Comparison of fluctuations of motor unit recruitment and de-recruitment thresholds in man. Exp Brain Res 95: 517–522, 1993 [DOI] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve 20: 679–690, 1997 [DOI] [PubMed] [Google Scholar]
- Semmler JG, Steege JW, Kornatz KW, Enoka RM. Motor-unit synchronization is not responsible for larger motor-unit forces in old adults. J Neurophysiol 84: 358–366, 2000 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Hayami A, Suzuki M, Watanabe S, Hutton RS. Reductions in recruitment force thresholds in human single motor units by successive voluntary contractions. Exp Brain Res 82: 227–230, 1990 [DOI] [PubMed] [Google Scholar]
- Tang P, Woollacott M. Phase-dependent modulation of proximal and distal postural responses to slips in young and older adults. J Gerontol A Biol Sci Med Sci 54: M89–M102, 1999 [DOI] [PubMed] [Google Scholar]
- Tax AA, Denier van der Gon JJ, Erkelens CJ. Differences in coordination of elbow flexor muscles in force tasks and in movement tasks. Exp Brain Res 81: 567–572, 1990 [DOI] [PubMed] [Google Scholar]
- Tax AA, Denier van der Gon JJ, Gielen CC, van den Tempel CM. Differences in the activation of m. biceps brachii in the control of slow isotonic movements and isometric contractions. Exp Brain Res 76: 55–63, 1989 [DOI] [PubMed] [Google Scholar]
- Theeuwen M, Gielen CC, Miller LE. The relative activation of muscles during isometric contractions and low-velocity movements against a load. Exp Brain Res 101: 493–505, 1994 [DOI] [PubMed] [Google Scholar]
- Tsuruike M, Koceja DM, Yabe K, Shima N. Age comparison of H-reflex modulation with the Jendrássik maneuver and postural complexity. Clin Neurophysiol 114: 945–953, 2003 [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Larsson L, Newell KM. Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity. Neurobiol Aging 24: 25–35, 2003 [DOI] [PubMed] [Google Scholar]
- Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve 25: 17–25, 2002 [DOI] [PubMed] [Google Scholar]
- Wolfarth S, Lorenc-Koci E, Schulze G, Ossowska K, Kamińska A, Coper H. Age-related muscle stiffness: predominance of non-reflex factors. Neuroscience 79: 617–628, 1997 [DOI] [PubMed] [Google Scholar]