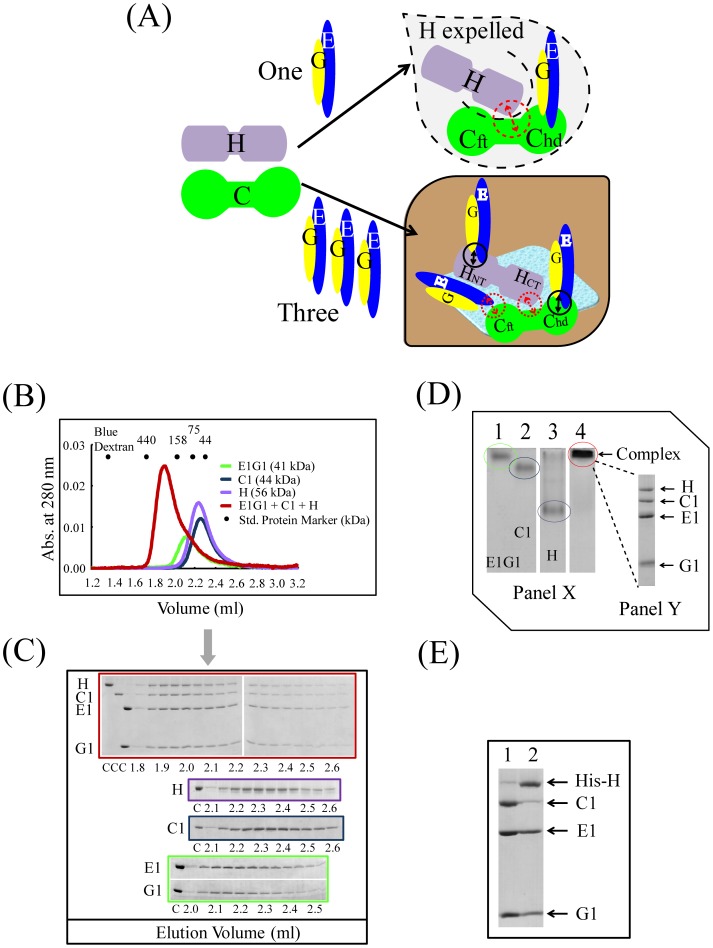
Figure 4. Ternary interactions of E1G1, C1, and H.
(A) Model of E1G1-C1-H assembly. Dotted arrows (red) and solid arrows (black) indicate weak and strong binding, respectively. (B) Gel filtration profile of the H/C1/E1G1 mixture (red) in comparison to H (purple), E1G1 (green), and C1 (blue) monomers. (C) SDS-PAGE analysis of the eluted fractions from gel filtration chromatography. Border colors indicate samples corresponding to the color scheme used in 4B. “C” indicates control proteins. (D) Panel X: Basic native polyacrylamide gel electrophoresis analysis of the H/C1/E1G1 mixture. A molar ratio of 3∶1∶1 of E1G1:C1:H proteins was prepared and incubated on ice for 1 h (lane 4). Bands corresponding to one molar amount of E1G1, C1, and H proteins are visible in lanes 1, 2, and 3, respectively. Panel Y: SDS-PAGE (12% gel) analysis of the E1G1-C1-H mixture band eluted from the native gel in panel X (lane 4). (E) SDS-PAGE of the eluted proteins from the His-tag pulldown experiment. Lane 1, fraction eluted using buffer B; lane 2, subunits bound with His-tagged H subunit eluted using buffer C.