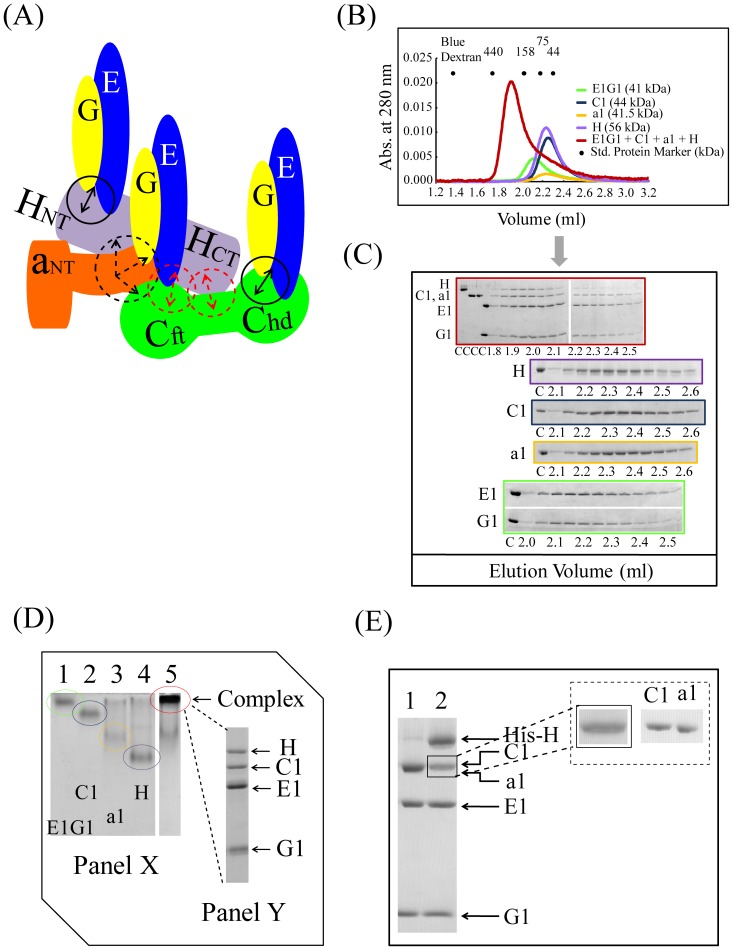
Figure 6. Quaternary interactions of E1G1, C1, a1NT, and H.
(A) Model of the quaternary subunit assembly. Dotted arrows indicate weak and solid arrows (black) strong binding. (B) Gel filtration profile of H/C1/a1NT/E1G1 mixture (red) in comparison to H (purple), C1 (blue), a1NT (yellow), and E1G1 (green) monomers. (C) SDS-PAGE analysis of the eluted fractions from gel filtration chromatography. Border colors indicate samples corresponding to the color scheme used in 6B. “C” indicates control proteins. (D) Panel X: Basic native polyacrylamide gel electrophoresis analysis of the H/C1/a1NT/E1G1 mixture. A 3∶2∶1∶1 molar ratio of E1G1:a1NT:C1:H proteins was prepared and incubated on ice for 1 h (lane 5). Bands corresponding to one molar amounts of E1G1, C1, a1NT, and H proteins are visible in lanes 1, 2, 3, and 4, respectively. Panel Y: SDS-PAGE (12% gel) analysis of the intense band eluted from the native gel in panel X (lane 5), suggesting the presence of C1, E1 and G1 in this complex. An unbound a1NT band was observed at the expected position. (E) SDS-PAGE of the eluted proteins from the His-tag pulldown experiment. Lane 1, fraction eluted using buffer B; lane 2, subunits bound with His-tagged H subunit eluted using buffer C.